Medtronic has issued a field safety notice over its Mazor X Surgical System, after reports that certain versions of the device had unexpectedly disconnected from operating tables having been securely attached.
Medtronic has issued a field safety notice over its Mazor X Surgical System, after reports that certain versions of the device had unexpectedly disconnected from operating tables having been securely attached.
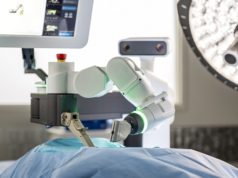
The Mazor X Surgical System is a robotic assisted spinal surgery platform which is raised and mounted over the operating room (OR) table, with the aid of an on-board automated lifting mechanism.
The Field Safety Notice, issued to UK healthcare professionals via the Medicines & Healthcare products Regulatory Agency (MHRA) in December, notes that the problem affects the Mazor X Surgical System (SST2) with Positioner Type II.
During pre-operative preparation, the Mazor X Surgical System is raised and mounted over the OR Table using a Manipulator automated lifting mechanism. This enables raising of the Surgical System over the OR table and mounting onto the Bed Frame. The Positioner provides a mechanism for rigid locking of the Surgical System on the OR table, after disconnecting it from the Manipulator
In its safety notice, the company said: “Medtronic is aware of instances where the Mazor X Positioner Type II has unexpectedly released from the OR table after being securely attached. Investigation has determined that the occurrences are due to the potential for slight air leakage over time within the pneumatic system resulting in a gradual decrease in the Positioner Type II latching device holding force. After time, this can result in the Surgical System to release from the Bed frame.”
The statement added that, as of 13 November 2019, Medtronic had received seven reports of the issue having occurred, but stressed that there had been no reports of patient injury associated with these incidents. No additional reports have been received since the letter was issued, a spokesperson for the company confirmed.
Medtronic said that it is working on a fix to permanently correct the issue—but has advised users that leaving the locking lever in an ‘open’ position will maintain pneumatic pressure within the system and keep the issue from occurring.
In a statement issued to Spinal News International, Medtronic said: “In December of 2019, Medtronic initiated a medical device correction related to the Mazor X Surgical System. The Mazor X is indicated for precise positioning of surgical instruments during general spinal surgery. It may be used in either open or minimally invasive or percutaneous procedures.
“Medtronic received complaints – none of which involved patient injuries – of the surgical system having issues maintaining a rigid connection to the operating room table. This could result in the surgical system slowly descending toward the user or patient.”
The company said reports are rare, but it has a mitigation to permanently correct the issue while offering instructions to all customers on how to prevent this from happening.
“The mitigation we offered to our customers resolves the issue entirely. We are now looking at long term product improvements. Our Medtronic Mazor X Surgical System representatives have been trained on this correction to the system and are present at every procedure that is performed on patients.
“At Medtronic, patient safety is our priority. We take this potential risk seriously, and we are working to ensure all Medtronic customers are fully aware of the risk and associated mitigations.”
The field safety notice can be found here: https://mhra-gov.filecamp.com/s/dnSgeTr3o3fdjN7n/d.