Tag: allograft
Orthofix announces first clinical use of Virtuos lyograft autograft substitute
Orthofix has announced the limited market release and first patient implant of the Virtuos lyograft, which the company describes as a first-of-its-kind, shelf-stable and complete...
i-FACTOR clinical results show higher fusion rate compared to allograft
Cerapedics has announced the publication of results from a clinical trial evaluating i-FACTOR Peptide Enhanced Bone Graft in non-instrumented lumbar fusion surgery in The...
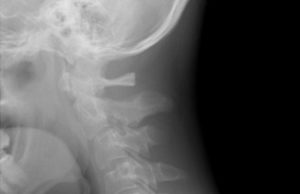
Cages in ACDF are associated with a higher non-union rate than...
Investigators found a higher rate of non-union associated with intervertebral cages than with allograft in a recent retrospective analysis, which led to the conclusion...
IVANOS study reveals i-FACTOR bone graft use more than doubles fusion...
Level 1 evidence, presented at EUROSPINE 2018 in Barcelona, Spain, demonstrates that i-FACTOR peptide enhanced bone graft results in significantly higher rates of fusion...
US FDA clears Xtant Medical’s new Calix-C cervical interbody footprints
The US Food and Drug Administration (FDA) has cleared Xtant Medical’s product line extensions for the Calix-C family of cervical interbody cages. The clearance...
Premier US healthcare improvement company to offer Bioventus’ full surgical portfolio
Bioventus has announced that its full surgical orthobiologics portfolio is now available through Premier, a US health care improvement company comprised of 3,750 US...
NASS’ allograft and spinal cord stimulation coverage recommendations open for public...
The president of the North American Spine Society (NASS), F Todd Wetzel (Philadelphia, USA), has announced the opening of the society’s 30-day public comment...
Burst Biologics initiates prospective multicentre clinical study in spinal fusion
Burst Biologics has received institutional review board approval to begin a multicentre prospective clinical study in spinal fusion patients. This study will be conducted...
DePuy Synthes launches ViviGen Formable cellular bone matrix
DePuy Synthes, in collaboration with LifeNet Health, has launched ViviGen Formable cellular bone matrix, a second-generation cellular allograft designed to assist in the formation...
LifeNet Health begins production of ViviGen cellular bone matrix at new...
LifeNet Health has begun production of ViviGen cellular bone matrix in its Northwest headquarters in Renton, USA. ViviGen, a cellular allograft developed by LifeNet...
Zimmer Biomet launches new PrimaGen advanced allograft
Zimmer Biomet has launched the PrimaGen advanced allograft. According to a press release, this autograft substitute offers the same bone-healing elements as autograft, but...
US patent granted to Theracell for demineralised bone fibre technology
The United States Patent and Trademark Office (USPTO) is to issue TheraCell a patent for its demineralised bone fibre (DBF) technology.
TheraCell's DBF technology is...
US FDA approves Spineology Rampart devices with allograft bone
Spineology has obtained US Food and Drug Administration (FDA)-clearance for the use of allograft bone with its Rampart Interbody Fusion Devices.
“This approval and our...
Spineology expands relationship with US Musculoskeletal Transplant Foundation
Spineology has expanded its relationship with Musculoskeletal Transplant Foundation (MTF), the USA’s leading tissue bank. MTF will now be the sole tissue provider for...