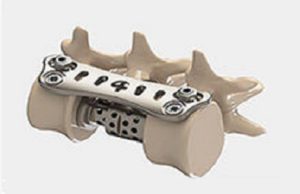
Tag: FDA clearance
Cutting Edge Spine receives FDA 510(k) clearance for EVOL ha-DLIF system
Cutting Edge Spine has received 510(k) clearance from the US Food and Drug Administration (FDA) for its EVOL ha-DLIF direct lateral interbody fusion system.
The...
SurGenTec announces FDA clearance for neurostimulation with Alara Access Needle Kit
According to a press release, SurGenTec has received FDA clearance for a neurostimulation indication for their ALARA Neuro Access Needle Kit. The ALARA system...
Medicrea announces FDA clearance of Tulip Genesis
The Medicrea Group announced today that it has received FDA clearance for Tulip Genesis, which completes its UNiD ASI platform technology.
With the FDA clearance...
Expanded FDA clearance for NuVasive’s Monolith corpectomy system
The Monolith corpectomy system (NuVasive) has been granted expanded FDA 510(k) clearance.
The system is now cleared for procedures in the cervical spine, between the...
FDA clears SpinalCyte IND application for universal donor cell therapy to...
The FDA has cleared SpinalCyte’s Investigational New Drug (IND) protocol for CybroCell. This is considered to be the first IND approval for a fibroblast...
Spinal Elements announces FDA clearance of Ti-Bond titanium coating as a...
Spinal Elements has recently announced FDA clearance for claims related to the macro-, micro-, and nano-surface structure of its Ti-Bond surface coating technology. Utilising this...
FDA approval granted for Eden Spine’s Sphynx
Eden Spine’s Sphynx plating system has been granted FDA clearance, the company has announced.
Sphynx was designed to complement the company’s Giza titanium vertebral body...
FDA clears first nanotechnology PEEK devices for spinal intervertebral fusion
The US Food and Drug Administration (FDA) has granted approval to market a polyetheretherketone (PEEK) spinal interbody fusion device with a PEEKplus nanotextured surface...
FDA clearance granted for Hive-C cervical interbody devices
HD LifeSciences has received FDA 510(k) clearance for its Hive-C IBFD, a system of interbody devices for anterior cervical fusion procedures.
The Hive-C implant system...
Renovis Surgical receives FDA clearance for Tesera Trabecular Technology Hyperlordotic ALIF...
Renovis Surgical Technologies has announced that it has received 510(k) clearance from the US Food and Drug Administration (FDA) to market the Tesera SA...
First patented single implant sacroiliac joint fusion system receives FDA clearance
The US Food and Drug Administration (FDA) have granted clearance for the Catamaran sacroiliac joint fixation system (Tenon Medical) specifically indicated for sacroiliac joint...
Bone cement solution indicated for treating sacral fractures granted FDA approval
The US Food and Drug Administration (FDA) has granted 510(k) clearance of Kyphon HV-R bone cement (Medtronic) for fixation of pathological fractures of the...
Paradigm Spine receives FDA pre-market approval (PMA) for first of its...
The US Food and Drug Administration (FDA) has granted pre-market supplemental approval (PMA) for the coflex interlaminar stabilisation disposable instrument kit (Paradigm Spine). This...
Rampart One standard ALIF interbody fusion system gains FDA clearance for...
The Rampart One Standard anterior lumbar interbody fusion (ALIF) device (Spineology) has been granted FDA clearance, allowing it to be used with or without...
FDA 510(k) clearance for cervical and lumbar HA PEEK interbody...
The US Food and Drug Administration (FDA) has granted 510(k) clearance for both the ALTA ACDF interbody spacers (Astura Medical) and HALF DOME lumbar...
Camber Spine announces 510(k) approval of ENZA-A titanium ALIF
The US Food and Drug Administration (FDA) has given 510(k) clearance to Camber Spine to market its ENZA-A titanium anterior lumbar interbody fusion (ALIF)...
Zimmer Biomet announces 510(k) clearance for Zyston strut open titanium interbody...
The US Food and Drug Administration (FDA) has given 510(k) clearance for the Zyston strut open titanium interbody spacer system (Zimmer Biomet). This marks...
Life Spine announces line extension of PRO-LINK titanium stand-alone cervical spacer...
The US Food and Drug Administration (FDA) has given 510(k) clearance to the PRO-LINK titanium stand-alone cervical spacer system (Life Spine) for spinal fusions.
PRO-LINK...
FDA approval for Infuse Bone Graft in expanded spinal surgery indications
Infuse Bone Graft (Medtronic) has gained US Food and Drug Administration (FDA) approval in new spine surgery indications. InfuseBone Graft is now approved for...