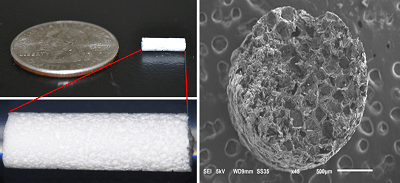
The INSPIRE study of InVivo Therapeutics’ Neuro-spinal scaffold—originally approved to enrol 12 patients—has been granted permission to expand to 20 patients, following the US Food and Drug Administration’s (FDA) review of six-month safety data from the first five patients implanted with the scaffold.
In addition to the 10 currently-implanted patients, two patients were screen failures, meaning that the patients consented to participate in the study, but failed to meet all of the inclusion and exclusion criteria of the study. Although these two patients were not implanted with the Neuro-spinal scaffold, they were technically enrolled into the study. Therefore, the study had enrolled 12 patients by the end of May (when the 10th patient was implanted by Domagoj Coric, chief of Neurosurgery at the Carolinas Medical Center, Charlotte, USA) and the enrolment of additional patients required action from the FDA.
After reviewing the six-month safety data, the FDA requested minor modifications to the INSPIRE study protocol and informed consent documents. These modifications are being incorporated, according to a press release, and the FDA has approved the enrolment of additional patients to allow for 20 evaluable patients (with six months of follow up data) in the INSPIRE study. Because of the frequent interactions with the FDA over the last several weeks regarding relatively minor modifications, InVivo chose to postpone updates on the INSPIRE study until there was clarity regarding the path forward. “We are pleased that the FDA has approved expansion of the INSPIRE study that clears the way for enrolling all 20 evaluable patients,” says Mark Perrin, InVivo’s chief executive officer and chairman. “While the pause in enrolment for the last several weeks was unfortunate, this approval is an important step toward our goal of approaching full enrolment of the INSPIRE study by the end of the year.”
During the interactions regarding the expansion of the INSPIRE study, the FDA also recommended that InVivo include a control arm in the study as part of a Study Design Consideration. Perrin said, “As is typical of the regulatory process, we have addressed a number of Study Design Considerations regarding the INSPIRE study and its pilot precursor study over the last two years. We have begun a constructive discussion with the FDA regarding this Study Design Consideration, and we will provide an update if substantial changes are made to the study protocol. We continue to believe that our current study design is sufficient to demonstrate safety and probable benefit in support of a Humanitarian Device Exemption (HDE) application for marketing approval. Given the encouraging results that we have observed to date, we look forward to working with the FDA to complete the INSPIRE study as efficiently as possible.”
Invivo Therapeutics has also received regulatory clearance in Canada. Health Canada has approved the company’s Investigational Testing Authorization application to commence clinical studies in Canada. The authorization will allow the company to enrol Canadian patients into the INSPIRE (InVivo Study of Probable Benefit of the Neuro-Spinal Scaffold for Safety and Neurologic Recovery in Subjects with Complete Thoracic American Spinal Injury Association Impairment Scale A Spinal Cord Injury) study once a Canadian site is open for enrolment.
Following this clearance, InVivo received approval from the Toronto Western Hospital’s Research Ethics Board (Toronto, Canada) to enrol patients as part of the INSPIRE study. Michael Fehlings has been named principal investigator at the site. Fehlings is currently director of the Spinal Program at Toronto Western Hospital at the University Health Network, professor in the Department of Surgery, full member of the Institute of Medical Sciences School of Graduate Studies, a scholar in the McLaughlin Centre of Molecular Medicine, a scientist in the McEwen Centre for Regenerative Medicine, a senior scientist at the Krembil Research Institute, director of the University of Toronto Neuroscience Program, co-director of the University of Toronto Spine Program, and Krembil chair in Neural Repair and Regeneration. Fehlings has published over 400 peer-reviewed articles, principally in the area of spinal cord injury and complex spinal surgery.
Fehlings says, “Spinal cord injury is a devastating condition for which innovative bioengineered regenerative strategies hold considerable promise. Accordingly, our centre is pleased to assist with translating this exciting technology for people with paralysis from spine trauma into the clinic.”













