 7D Surgical has received both 510(k) clearance from the US Food and Drug Administration (FDA) and a medical device license from Health Canada, enabling the North American commercial launch of its Machine-vision image guided surgery (MIGS) system for spine surgery.
7D Surgical has received both 510(k) clearance from the US Food and Drug Administration (FDA) and a medical device license from Health Canada, enabling the North American commercial launch of its Machine-vision image guided surgery (MIGS) system for spine surgery.
The 7D surgical system employs 3D optical technologies and machine vision algorithms to eliminate the long-standing barriers to adoption of existing surgical navigational platforms. This technology is designed to easily register spinal surgery patients automatically using only visible light. Unlike time-consuming conventional image guided surgery systems that depend on intraoperative radiation, this new platform can achieve an incredibly fast surgical workflow for spine procedures.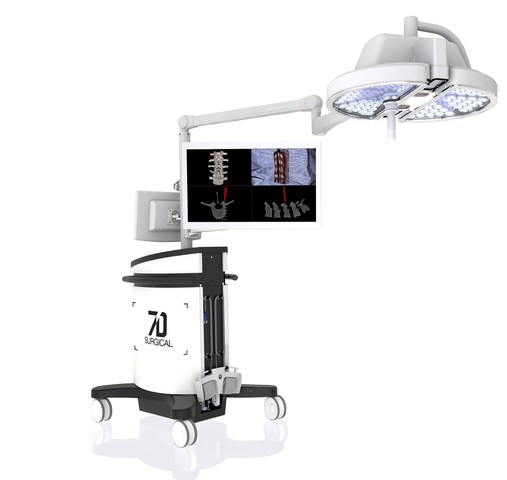
The MIGS navigation technology is embedded in an on-board overhead surgical light, which is designed to eliminate line-of-sight frustrations in the operating room which is controlled by the surgeon using only a foot pedal.
“Image-guided surgery technology has finally caught up to the needs of a practicing spine surgeon,” says Frank Cammisa, chief emeritus of the Spine Service at Hospital for Special Surgery in New York City, USA. “7D Surgical’s new MIGS system appears to provide a faster, radiation-free alternative to existing options. It could be an important new tool in expanding the use of image-guided surgery in spine procedures.” He adds, “The 7D Surgical system reduces the overall cost and footprint required to navigate the spine. It is a win-win for the surgeon and the hospital.”













