Tag: US FDA clearance
Wenzel Spine receives US FDA clearance expanding clinical indications for primaLOK...
Wenzel Spine has gained US Food and Drug Administration (FDA) clearance for its primaLOK SP system—marking a "significant advancement in spinal fusion technology", offering...
FDA approval for M6-C artificial cervical disc
The US Food and Drug Administration (FDA) has approved the M6-C artificial cervical disc (Orthofix Medical) for patients suffering from cervical disc degeneration. The...
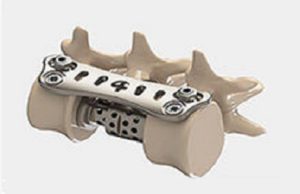
FDA 510(k) clearance for Astura Medical’s spinal fixation system
FDA 510(k) clearance has been granted for the Olympic minimally invasive surgery (MIS) posterior spinal fixation system (Astura Medical).
The Olympic MIS system delivers a...
FDA approval granted for Eden Spine’s Sphynx
Eden Spine’s Sphynx plating system has been granted FDA clearance, the company has announced.
Sphynx was designed to complement the company’s Giza titanium vertebral body...
FDA clears first nanotechnology PEEK devices for spinal intervertebral fusion
The US Food and Drug Administration (FDA) has granted approval to market a polyetheretherketone (PEEK) spinal interbody fusion device with a PEEKplus nanotextured surface...
FDA clearance granted for Hive-C cervical interbody devices
HD LifeSciences has received FDA 510(k) clearance for its Hive-C IBFD, a system of interbody devices for anterior cervical fusion procedures.
The Hive-C implant system...
Renovis Surgical receives FDA clearance for Tesera Trabecular Technology Hyperlordotic ALIF...
Renovis Surgical Technologies has announced that it has received 510(k) clearance from the US Food and Drug Administration (FDA) to market the Tesera SA...
First patented single implant sacroiliac joint fusion system receives FDA clearance
The US Food and Drug Administration (FDA) have granted clearance for the Catamaran sacroiliac joint fixation system (Tenon Medical) specifically indicated for sacroiliac joint...
Bone cement solution indicated for treating sacral fractures granted FDA approval
The US Food and Drug Administration (FDA) has granted 510(k) clearance of Kyphon HV-R bone cement (Medtronic) for fixation of pathological fractures of the...
icotec receives FDA clearance to market BlackArmor® Carbon/PEEK interbody cages
The latest line of icotec interbody cages, designed to optimise bony integration and post-operative visualisation, has received US Food and Drug Administration (FDA) 510(k)...