Connecticut Orthopaedic Specialists have announced the enrollment of the initial patients within Simplify Medical’s clinical trial to evaluate the Simplify Disc, its novel investigational cervical disc, at two adjacent cervical levels. James J Yue, a spine and neck specialist at Connecticut Orthopaedic Specialists, is leading the trial site as a study investigator.
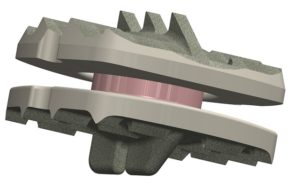
The Simplify Disc is a titanium-coated, PEEK on ceramic, anatomically-designed, biocompatible artificial cervical disc designed specifically for motion preservation and diagnostic imaging optimisation. The two-level IDE trial, designed to investigate the clinical efficacy of the disc, is a prospective, multi-centre, clinical trial evaluating the disc at 18 clinical sites in the USA. The trial compares the disc with anterior cervical discectomy and fusion (ACDF) as a historical control.
Yue comments, “Successful treatment allowing for motion preservation and continued diagnostic imaging potentially without exposure to radiation following implantation is extremely important to our patients. The Simplify Disc is an investigational, novel option for cervical arthroplasty and we look forward to working with Simplify Medical to evaluate this technology.”
Yue continues, “In addition to its motion preserving design and non-metal articulating components, which allow for MRI diagnostic imaging, the Simplify Disc is also designed with a unique disc height option to better match anatomical cervical disc heights, especially in women, and with durable materials that mitigate adverse metal wear. Our participation in this trial exemplifies the dedication of physicians at Midstate Medical Center, the Connecticut Orthopaedic Institute and Connecticut Orthopaedic Specialists to motion-sparing, minimally-invasive, non-fusion surgical procedures of the spine for over two decades.”
In a previous Simplify Medical clinical trial, despite the predecessor device under investigation demonstrating superiority over fusion at one level for all patients, men did statistically better than women. Speaking exclusively to Spinal News International recently, Simplify Medical CEO David Hovda explains, “We did a lot of research into what could possibly cause a difference in outcome based on gender. We concluded that our lowest height, in retrospect, was too tall. Women were getting their disc space relatively more distracted than men, which we believed was leading to the inferior outcome.” According to the literature, average cervical disc height is approximately 4mm; the smallest cervical disc offered by Simplify Medical was 5.7mm at that time.
Hence, Simplify Medical now offer a 4mm device; early results are promising. Hovda expands, “We felt that it would be better to make lower height devices that better match the anatomy, rather than distracting the anatomy to match the fusion spacer height. We are seeing, although it is still early days, compelling clinical outcomes which we think are potentially attributable to the fact that we have a lower height which better matches the patient anatomy.”
Enrollment for this clinical trial is expected to be complete by the end of 2018.













