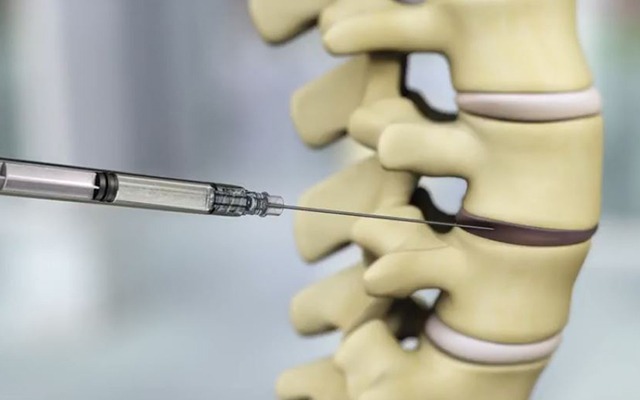
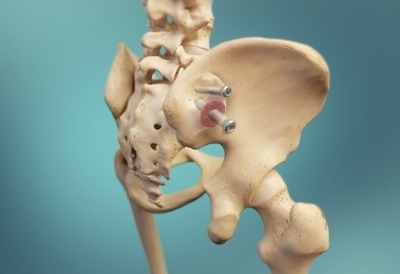
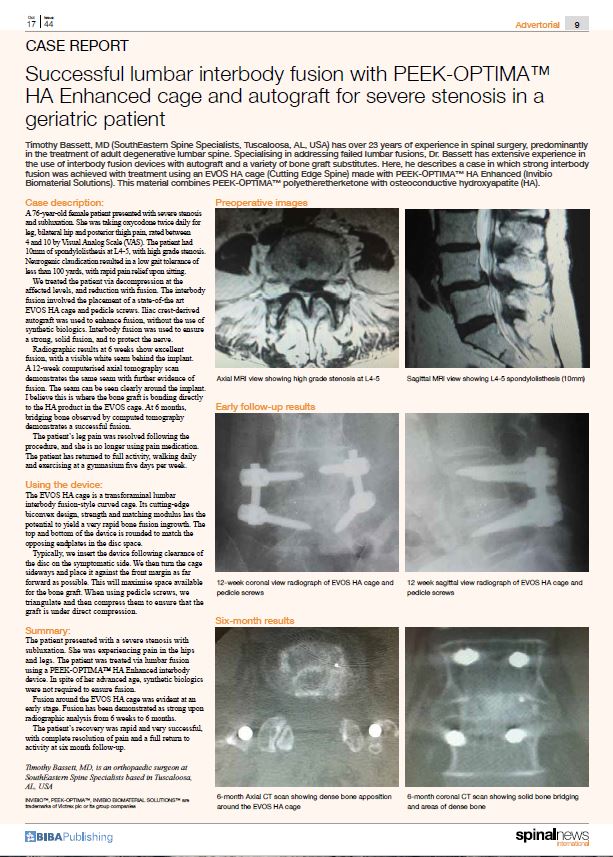
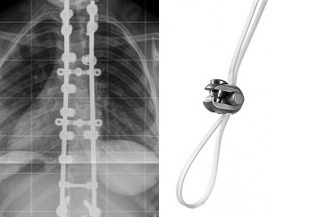
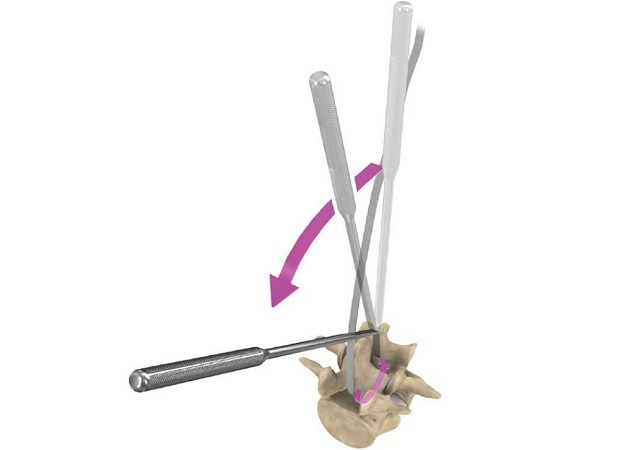
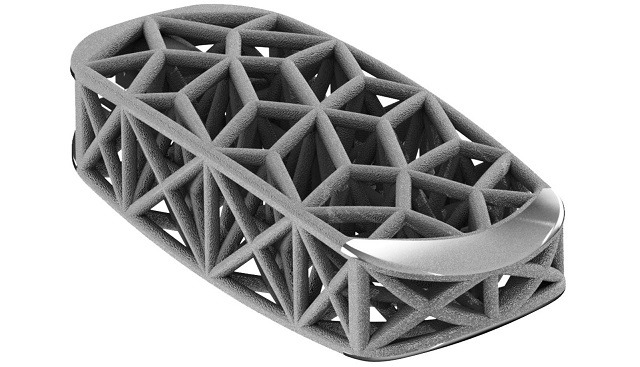
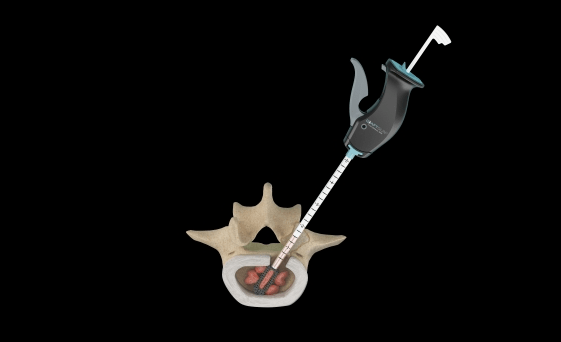
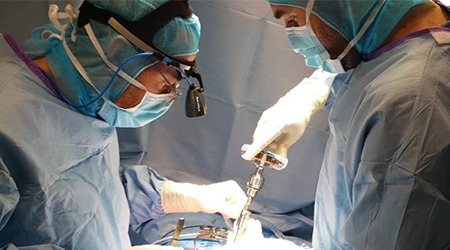
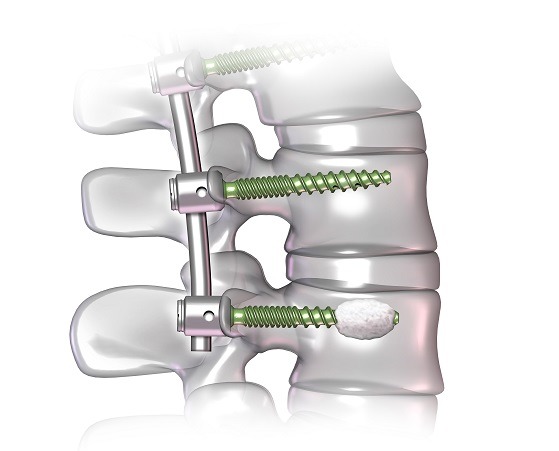
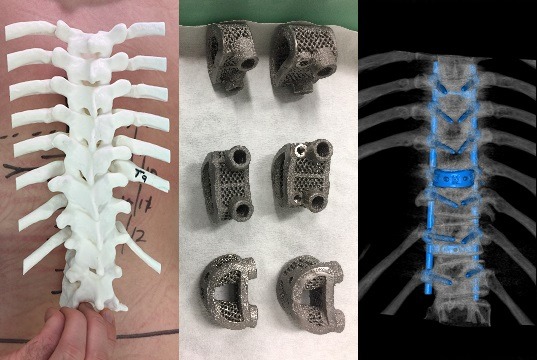
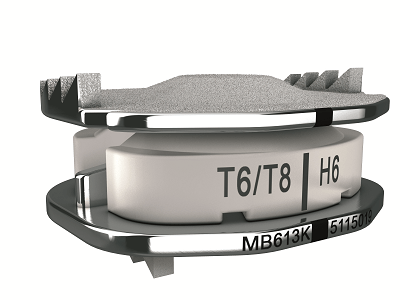
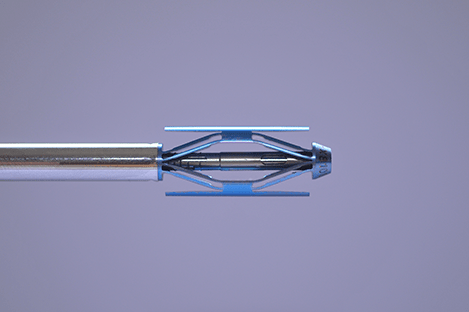
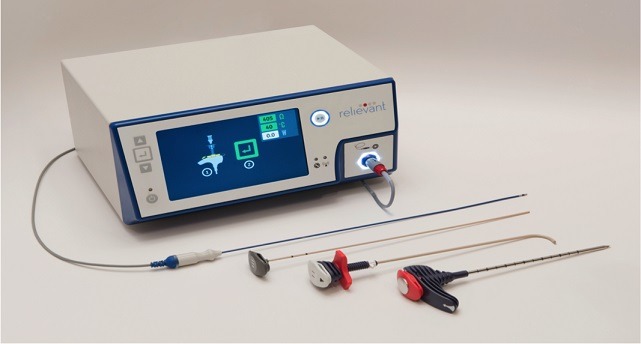
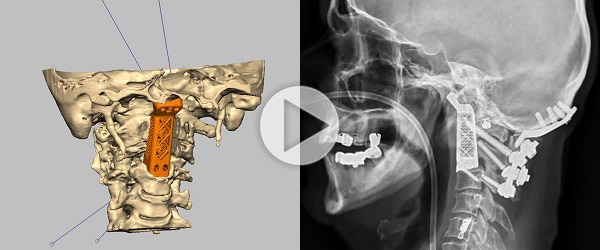
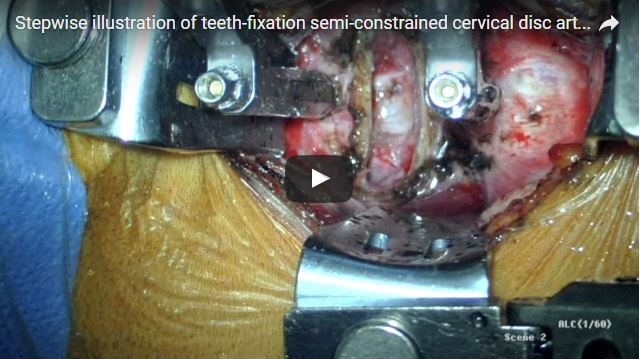
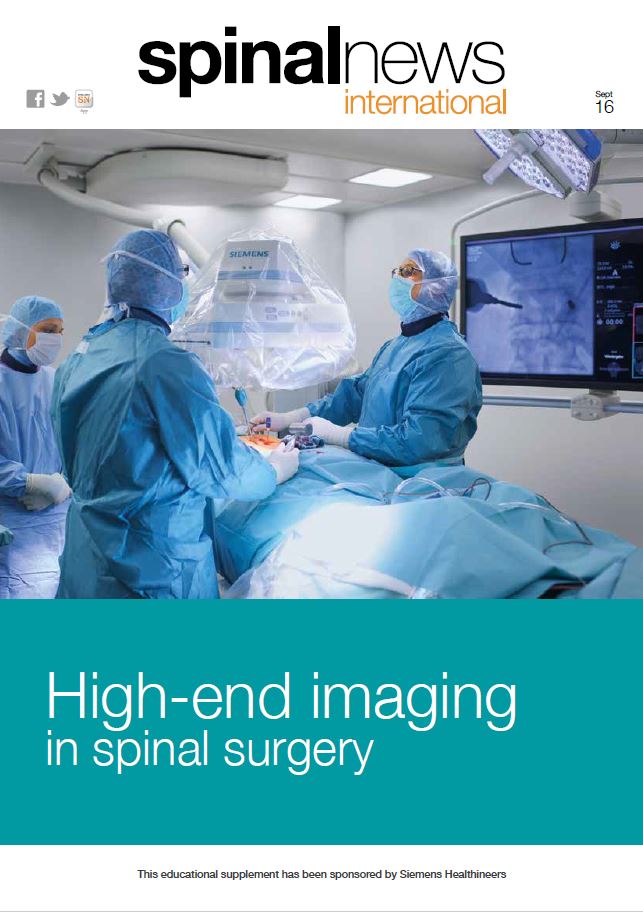
Relievant Medsystems recently announced the publication of the Level I INTRACEPT Study results in The Spine Journal. The INTRACEPT Study is a Level I, randomised, multicentre trial comparing the Intracept Procedure to non-surgical standard care. Based on the results...
Biogen today announced new results from the NURTURE study, adding data to the longest study of spinal muscular atrophy (SMA) in pre-symptomatic infants (n=25). These data reported, after up to 45.1 months of analysis, continue to demonstrate efficacy and...
The NSpine Main Conference will focus on the entire spine during a series of lectures & workshops. We comprehensively cover the entire range of spinal conditions relevant to spine health care professionals. Stay tuned.... We go live on Monday...
Medtronic today announced it has completed the acquisition of Titan Spine, a privately-held titanium spine interbody implant and surface technology company. A definitive acquisition agreement between the two companies was previously announced on 9 May 9 2019. The acquisition...
Fusion Robotics today announced the closing of a financing round for an undisclosed amount with lead investment by Alex Lukianov (Lukpartners) and Kevin Foley. The proceeds will be used to ready the company's spinal robotics platform to obtain regulatory clearance...
To enable upcoming regulatory filings, Medtronic has made public its partnership with Karl Storz, who have developed a system for endoscopic imaging. For the past four years, a press release reports, the two companies have partnered to seamlessly integrate...
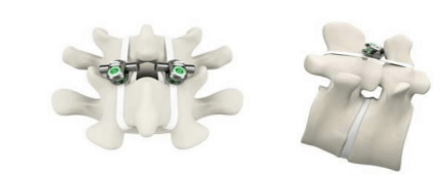
Life Spine has announced that the US Food and Drug Administration (FDA) has provided 510(k) market clearance for the ProLift lateral expandable spacer system.
“The ProLift lateral expandable spacer system is a significant addition to Life Spine’s rapidly growing expandable...
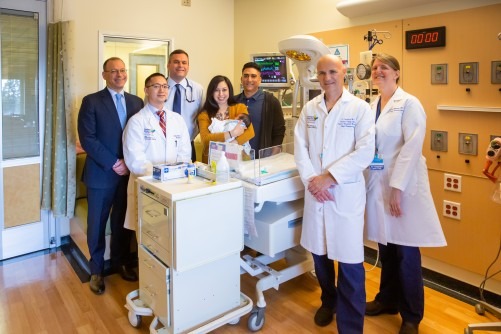
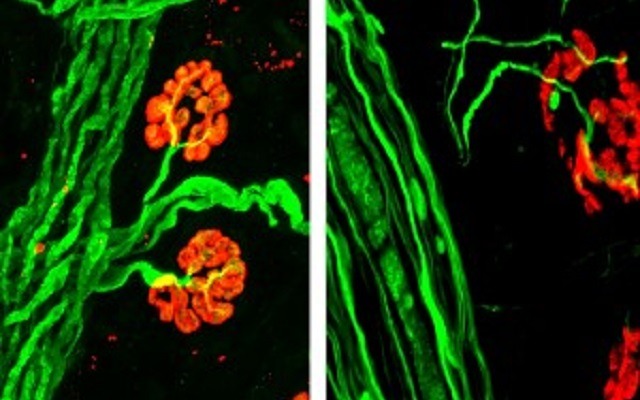
Gilda Giron was just 13 weeks pregnant when an ultrasound revealed her baby had myelomeningocele—the most severe form of open spina bifida, a birth defect that affects backbone development and can cause, among other things, debilitating neurological damage.
“This was...
NYU Langone Health last month became the first centre in the USA to perform a discectomy with repair of a large annular defect using a titanium bone-anchored implant newly approved by the US Food and Drug Administration (FDA). The...
Boston Scientific has announced the close of its acquisition of Vertiflex, a privately-held company that developed and commercialised the Superion Indirect Decompression System, a minimally-invasive device used to improve physical function and reduce pain in patients with lumbar spinal stenosis...
Spinal neurosurgeon Todd H Lanman has become the first US doctor to perform a two-level artificial disc replacement surgery using the newly US Food & Drug Administration (FDA) approved M6-C device from Orthofix. He performed the procedure with his...
RTI Surgical today announced a milestone of 5,000 implants of Fortilink-C, -TS and -L interbody fusion (IBF) systems with TETRAfuse 3D technology in the USA.
“In my over 500 implantations, I have been extremely satisfied with the Fortilink-TS and -C...
Implanet has announced successful results of the first surgeries using Jazz Cap in the USA. Following 510(k) clearance in March 2019, the first Jazz Cap procedures have been successfully completed by fellowship trained orthopaedic spine surgeon Chi Lim, in...
Medacta International recently announced its MySpine Midline Cortical (MC) platform has been recognised as this year’s “Best Healthcare Navigation / Robotics Solution” by MedTech Breakthrough. MySpine MC is the Medacta’s patient-matched, 3D printed solution in the midline cortical approach,...
RTI Surgical today announced MedTech Breakthrough has selected TETRAfuse 3D technology as the “Best New Technology Solution - Orthopedics” in their 2019 Awards Program. MedTech Breakthrough is an independent organisation recognising the top companies and solutions in the global...
Astura Medical and Academy Medical recently announced their partnership for contracting within the Department of Defense (DoD) and VA.
According to a press release, Academy Medical’s goal is to provide sales channels, via contracts, to appropriate medical and surgical vendors,...
Collegiate football players have low rates of serious or disabling injuries of the cervical spine, concludes an analysis of a National Collegiate Athletic Association (NCAA) database, reported in the journal Spine. The journal is published in the Lippincott portfolio...
Misonix today announced that it received 510(k) clearance by the US Food and Drug Administration (FDA) for Nexus, its ultrasonic surgical platform. Misonix will commence the commercialisation of the Nexus platform in the USA in July.
Nexus is an integrated...
Results of the CSM-Protect trial show that a six-week course of riluzole as an adjunct to surgical decompression for moderate-severe degenerative cervical myelopathy (DCM) has “no significant benefit” to the primary outcome of mJOA and “interesting effects” on three...
Globus Medical recently announced the launch of AERIAL, a minimally invasive expandable interspinous fixation system with independent locking plates. The expandable central core of AERIAL provides continuous distraction for indirect decompression and a customised patient fit. This is Globus...
Alphatec Holdings recently announced the commercial release of its IdentiTi-PC porous titanium interbody implant system for transforaminal lumbar interbody fusion procedures (TLIF).
“The commercial launch of IdentiTi-PC demonstrates continued solid progress against our commitment to accelerate growth by compelling the...
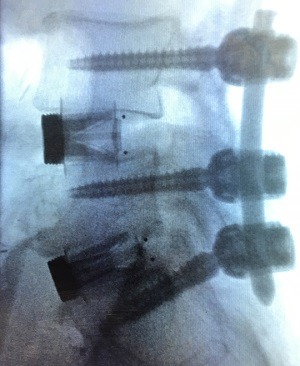
According to a recent study, the low-virulent microorganisms frequently detected on pedicle screws by using sonication may be an important cause of implant loosening and failure. Additionally, the investigators found that a longer surgical duration increases the likelihood of...
Marios Papadopoulos and Samira Saadoun write in Spinal News International about the ISCoPE trial, which aimed to develop techniques to continuously monitor the pressure of the spinal cord at the injury site in the intensive care unit (ICU). They...
A clinical professor of neurosurgery, Ronald Bartels talks to Spinal News International about his career. From his days at medical school to his appointment as chair of the Department of Neurosurgery at Radboud University Medical Center, he discusses how...
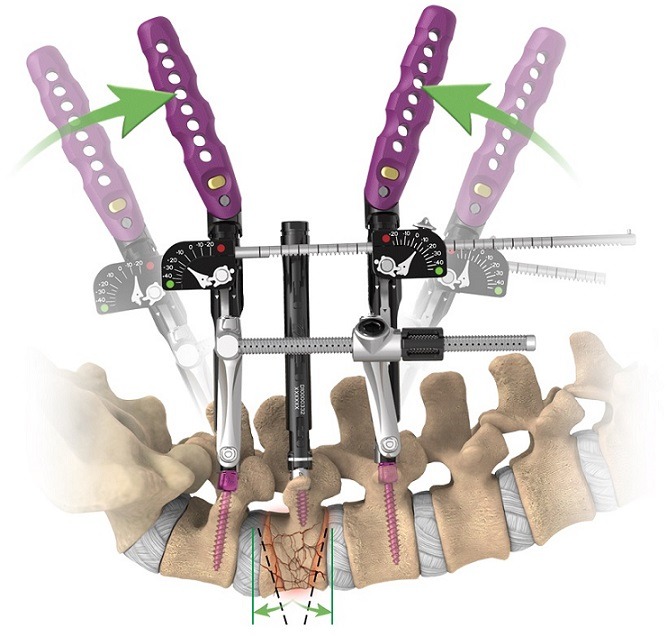
Globus Medical has announced the results of a clinical study that evaluated the versatility of ExcelsiusGPS robotic navigation system compared to traditional pedicle screw placement techniques. Published in the Journal of Robotic Surgery, “Robotic-assisted navigated minimally invasive pedicle screw...
EOS imaging has announced its fourth EOS system installation at the Hospital for Special Surgery (HSS; New York City, USA). HSS is the world’s largest academic medical centre dedicated to musculoskeletal health, performing more than 33,000 surgical procedures a...
Results of a worldwide survey indicate that the overall prevalence of burnout among spine surgeons is 30.6%. They suggest that factors linked with higher likelihood of burnout include working in North America, being in training as a fellow, and...
The first study to describe spinal trauma management and its outcomes in East Africa was recently published in the Journal of Neurosurgery: Spine. First authors Andreas Leidinger and Eliana E Kim, senior author Roger Härtl (Weill Cornell Brain and...
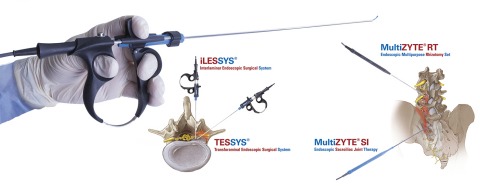
Joimax is showcasing its integrated navigation tracking and control system, Intracs, at this year’s Global Spine Congress (GSC; 15–18 May, Toronto, Canada). The company is also promoting the system at the annual meeting of the German Society of Neurosurgery...
Kaia Health has announced results from the first randomised controlled trial of its app-based therapy program for patients with non-specific low back pain. Detailed results of the study were published in the journal NPJ Digital Medicine and showed that...
Outgoing British Association of Spine Surgeons (BASS) president, Stuart Blagg (Aylesbury, UK), sits down with incoming president Sashin Ahuja (Cardiff, UK) at the BASS 2019 conference in Brighton to discuss Blagg’s achievements over the past two years as well...
Medtronic recently announced that it has entered into a definitive agreement pursuant to which it will acquire Titan Spine, a privately-held titanium spine interbody implant and surface technology company. The boards of directors of both companies have unanimously approved...
Boston Scientific today announced that it has entered into a definitive agreement to acquire Vertiflex, a privately-held company which has developed and commercialised the Superion Indirect Decompression System, a minimally-invasive device used to improve physical function and reduce pain...
Highlights:
Cages in ACDF are associated with a higher non-union rate than allograft
Advanced method of quantifying the cone of economy may allow practitioners to determine appropriate treatment options
While the ASBMR taskforce report concludes that “no further trials...
Nicholas Theodore (Baltimore, USA) talks to Spinal News International about the benefits of robotics technology in spinal surgery including reducing radiation exposure to surgeons, improving accuracy and precision and ultimately allowing patients to get better faster. Theodore looks at...
At the 2019 meeting of the International Society for the Advancement of Spine Surgery (ISASS; 3–5 April, Anaheim, USA), Erik Wang of NYU Langone Health (New York, USA) presented the results of a study reviewing the rates of hospital-acquired...
Investigators found a higher rate of non-union associated with intervertebral cages than with allograft in a recent retrospective analysis, which led to the conclusion that allograft may be superior to cages in anterior cervical discectomy and fusion (ACDF). This...
As the incoming president of the Spine Society of Australia, Matthew Scott-Young details how his experience as an Emergency Department intern sparked a life-long interest in spine care. He addresses current challenges, warning against the dangers of cost minimisation,...
Relievant Medsystems have announced the publication of 24-month results from the SMART trial in the International Journal of Spine Surgery. A total of 106 of the 128 treatment arm patients in the SMART trial completed 24-month follow up. Data...
Orthofix Medical today announced the first commercial implants of patients with the M6-C artificial cervical disc. The Center for Disc Replacement at Texas Back Institute (TBI) in Dallas, USA recently implanted four patients suffering from single-level cervical disc degeneration...
Winner of the Sanford J Larson Award, Nathan Xie, presented his research, "Use of artificial intelligence to improve surgical referrals in degenerative lumbar spine conditions", during the 2019 American Association of Neurological Surgeons Annual Scientific Meeting (AANS; 13–17 April,...
EOS imaging today introduced EOSlink, the company's new solution enabling the seamless integration of its EOSapps preoperative surgical planning software with intraoperative surgical solutions, such as navigation devices, robotics-based systems and custom spinal rod solutions.
EOSapps is EOS imaging's suite...
A recent study conducted by Peter G Passias (NYU Langone Health, New York Spine Institute, New York, USA) and colleagues showed that male patients and patients with increased comorbidity severity experienced a greater length of hospital stay (LOS) after...
A new method to quantify the boundaries of Dubousset’s cone of economy, the centre of mass displacements, and the amount of sway within the cone of economy along with the energy expenditure for a specific patient, has been developed...
A recent taskforce report charged by the American Society for Bone and Mineral Research (ASBMR) concluded that current evidence does not support the routine use of vertebroplasty for the treatment of pain from vertebral fractures. However, as someone who...
A recent study found that a longer duration of preoperative radiculopathy symptoms in patients with degenerative cervical pathology is associated with worse health-related quality of life (HRQOL) outcome measures after 1–3 level anterior cervical discectomy and fusion (ACDF) surgery....
Optimal correction of sagittal alignment and improving a patient’s functional capacity can significantly influence postoperative frailty resolution, according to a recent study by Peter Passias (NY Spine Institute, New York, USA) and colleagues. According to the authors, the CD-FI...
Francis Lovecchio (Hospital for Special Surgery , New York, USA) recently gave a presentation on opioid consumption patterns after lumbar microdiscectomy or decompression at the 19th Annual Conference of the International Society for the Advancement of Spinal Surgery (ISASS;...
A recent study found that low back pain is significantly improved in 74% of patients immediately after surgery for lumbar spinal stenosis and that at two years, over two-thirds of patients continue to have significant relief of low back...
At the annual meeting of the International Society for the Advancement of Spinal Surgery (3–5 April, Anaheim, USA), Jack E Zigler (Texas Health Center for Diagnostics and Surgery, Texas Back Institute, Plano, USA) reported results which “strongly support” the...
Spineology has announced that two abstracts highlighting the company’s proprietary mesh technologies were presented during the 19th Annual Meeting of the International Society for the Advancement of Spine Surgery (ISASS; 3–5 April, Anaheim, USA).
In a poster titled "Patient-reported outcomes...
Highlights:
Single-level anterior cervical discectomy provides "solid alternative" to fusion or arthroplasty
Banned in the USA: Petition calls for FDA to prohibit reprocessed pedicle screws
N20: Spine in the spotlight at global meeting
Expanding access to surgery on a...
Orthofix Medical today announced the full two-year outcomes from its US Investigational Device Exemption (IDE) study of the M6-C artificial cervical disc (Orthofix).
Jack Zigler, orthopaedic spine surgeon at Texas Back Institute (Plano, USA) and an investigator in the study,...
New retrospective studies led by Charla Fischer, associate professor of orthopaedic surgery and director of quality and patient safety at NYU Langone’s Spine Center (New York, USA), show that the number of opioids taken before and during hospital stays...
Anterior cervical discectomy and fusion (ACDF), can be safely performed in an outpatient setting in select patients, according to a study at Hospital for Special Surgery (HSS; New York, USA).
The research, titled, “A comparison of multilevel anterior cervical discectomy...
EOS imaging has announced installation of the first EOS system in the United Arab Emirates (UAE), located at the newly inaugurated King's College Hospital (Dubai, UAE) in January 2019.
The new hospital, a joint venture of the Al Tayer Group,...
Eric Lamoutte and Kern Singh (Department of Orthopaedic Surgery, Rush University Medical Center, Chicago, USA) discuss results of their recent study examining whether or not day of surgery affects length of stay and hospital charges following lumbar decompression. While...
It was recently announced that RTI Surgical has enrolled the first patient in its "Clinical evaluation of Fortilink interbody fusion device with TETRAfuse 3D technology in subjects with degenerative disc disease" (FORTE) study.
FORTE is a prospective, multicentre post-market evaluation of...
Current evidence does not support the use of vertebroplasty for the treatment of pain from vertebral fractures, concludes a recent task force report charged by the American Society for Bone and Mineral Research (ASBMR) aimed at examining the efficacy...
SpinalCyte recently announced the issuance of new patents in the USA and Japan. The company’s patent portfolio now includes 39 US and international patents issued with over 100 patents pending focused on the clinical use of fibroblasts.
The company...
A study at the Hospital for Special Surgery (HSS; New York, USA) found that a CT scan of the lumbar spine prior to surgery indicated that a significant number of patients had low bone density that was previously undiagnosed....
RTI Surgical has announced it has completed the acquisition of Paradigm Spine.
Paradigm Spine's primary product is the Coflex interlaminar stabilisation device. According to a press release, this is a “differentiated, minimally invasive motion preserving stabilisation implant that is FDA...
This advertorial has been sponsored by INVIBIO ™
Hyun W Bae, MD, is a medical director at The Spine Institute (Saint John’s Health Center, Santa Monica, USA) and professor of Surgery at Cedars-Sinai Medical Center (Los Angeles, USA). An orthopaedic...
Inspired Spine recently celebrated the grand opening of its concierge centre with a two day event that included a cadaver training lab at its Burnsville Total Spinal Health Center. The 120,000 sq. ft. three building campus is a testament...
The commissioner of the US Food and Drug Administration (FDA), Scott Gottlieb, has unexpectedly resigned after serving just shy of two years in the post.
Gottlieb announced his intention to stand down in a letter to Alex Azar II, the...
Two-year results of a randomised controlled trial, published in the Journal of Bone and Joint Surgery, suggest that minimally invasive sacroiliac joint arthrodesis with triangular titanium implants is safe and more effective throughout two years in improving pain, disability, and...
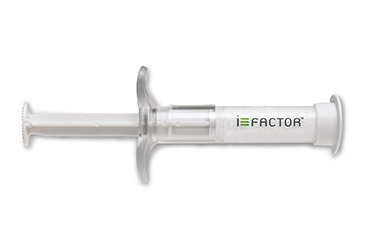
The International Society for the Advancement of Spine Surgery (ISASS) recently issued a new bone grafting policy that features i‑FACTOR peptide enhanced bone graft (Cerapedics) as one of only two drug-device combination products approved by the US Food &...
Vertos Medical said it has received CE mark approval for its lumbar spinal stenosis (LSS) treatment device kit.
According to a press release, the company's Mild device kit enables a minimally invasive procedure to remove the cause of stenosis through a...
Universal health is a top priority at the Weill Cornell Brain and Spine Centre in New York, USA. Their global neurosurgery programme, a collaborative project designed to improve access to surgery and surgical care across the world, has been...
Results of a double-blinded randomised controlled trial indicate that anterior cervical disc arthroplasty (ACDA) does not lead to a superior outcome in comparison to anterior cervical discectomy with fusion (ACDF) or anterior cervical discectomy (ACD) alone. Subsequent investigation involving...
Atlas Spine has announced the successful completion of its 50th surgical procedure and over 100 devices implanted with its new HiJak AC expandable cervical interbody fusion device. The company has now moved to its full product launch.
According to a...
OrthoPediatrics has announced the launch of BandLoc DUO, the latest addition to the BandLoc 5.5/6.0mm system. BandLoc is a temporary implant for use in orthopaedic surgery, intended to provide stabilisation as a bone anchor during the development of solid bony fusion...
It was announced recently that Alphatec has received 510(k) clearance from the US Food & Drug Administration (FDA) for its automated SafeOp neuromonitoring system for use in real-time intraoperative nerve location and health assessment.
According to a press release, the technology of...
A recent study found that patients who presented with presurgical depressive symptoms reported more severe symptoms preoperatively and postoperatively. However, despite residual symptoms, these patients may benefit more from surgery than those without depressive symptoms. The results were presented...
Inspired Spine's H Abbasi has released a study presenting clinical, and radiological outcomes including fusion rates for oblique lateral lumbar interbody fusion (OLLIF). The study establishes, based upon a 300 plus patient population, that OLLIF is an extraordinary, safe,...
Life Spine has announced the initiation of the SIMPACT sacroiliac joint (SI) fixation outcomes study, with Keith Maxwell of Southeastern Sports Medicine and Orthopedics in Asheville, USA.
SIMPACT is a cannulated and fenestrated screw, intended for sacroiliac joint fusion for...
While non-specific low back pain (NSLBP) is a common diagnosis, Tim Germon (Derriford Hospital, Plymouth, UK) argues that most low back pain is in fact specific. He speaks about the importance of reaching a diagnosis in order to drive...
A new study has found that after cervical decompression surgery, cervical spondylotic myelopathy (CSM) patients exhibited improved gait pattern, spatiotemporal parameters, spine and lower extremity range of motion (ROM), and patient reported outcomes. The study was carried out by...
Researchers at Johns Hopkins Medicine (Baltimore, USA) report that a computer programme they designed may help surgeons identify and label spinal segments during real time operating room procedures and avoid the costly and potentially debilitating consequences of operating on...
The US Food and Drug Administration (FDA) has approved the M6-C artificial cervical disc (Orthofix Medical) for patients suffering from cervical disc degeneration. The artificial disc was developed by Spinal Kinetics, a company acquired by Orthofix in April 2018.
The...
Southern New Hampshire Medical Center has installed the 7D Surgical System for spinal procedures. This system virtually replaces standard fluoroscopy, providing the surgical team with a fast, accurate and radiation-free tool for the placement of spinal implants.
The 7D Surgical System...
Sirakoss has been granted CE mark clearance in the European Union (EU) for Osteo3, a novel nanosynthetic bone graft substitute designed to improve patient healing, offering surgeons a more advanced solution for repairing bone fractures.
Based on proprietary nanoporous technology,...
Three new StabiLink Dual Lamina Implants (Southern Spine) have been released. Alan H Daniels, associate professor of Orthopaedic Surgery at The Warren Alpert Medical School of Brown University (Providence, USA) has specifically used the StabiLink Dual Lamina constructs as...
A novel enhanced recovery after surgery (ERAS) protocol developed by Penn Medicine (Philadelphia, USA) for patients undergoing spinal and peripheral nerve surgery significantly reduced opioid use. A new study published in the Journal of Neurosurgery: Spine showed that when an ERAS protocol was...
The editors-in-chief of major cardiovascular journals—of both US and European societies—have come together to “sound the alarm” about the dangers of medical misinformation that has been disseminated through the internet, social media, and other platforms. They claim that this...
The Secure-C cervical artificial disc (Globus Medical) is now covered by Anthem, one of the largest health benefits companies in the United States with close to 40 million medical members and over 73 million lives covered.
Secure-C is designed for...
A new patent has been issued by the United States Patent and Trademark Office (USPTO) for United States Patent No. 10,143,501 entitled “Expandable Interspinous Device”. This new patent relates to Aurora Spine’s family of minimally invasive spinal implants, bolstering...
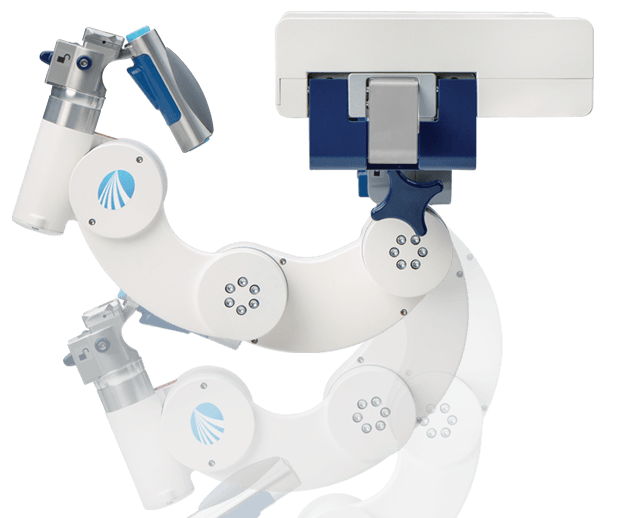
The first US patients have been treated with the Mazor X Stealth Edition (Medtronic) for spine surgery following its commercial launch. The technology offers a procedural solution for surgical planning, workflow, execution and confirmation. The system was first used...
Daniel Spencer is a business manager at Charlton Morris, an executive search firm specialising in orthopaedics, spine and simulation. He argues that while adoption of virtual reality and simulation represents a “leap of faith” for hospitals, the technology has...
Bioventus has launched OSTEOMATRIX+, a biphasic bone graft for use in bone remodelling in a variety of orthopaedic and spine applications. OSTEOMATRIX+ is a mouldable bone graft substitute consisting of bovine collagen and biphasic, hydroxyapatite/ß-tricalcium phosphate granules designed to produce a porous scaffold...
Back pain app creator, Kaia Health, today announces it has raised US$10 million in a Series A round led by Balderton Capital. The investment will be used to support Kaia Health’s US rollout, including a new office in New...
The US government shutdown means the country’s Food and Drug Administration (FDA) cannot accept new user fees, which means the agency cannot accept new medical product applications.
FDA commissioner Scott Gottlieb took to Twitter to highlight agency employees who are...
Mighty Oak Medical has received CE mark clearance for its patient-specific, 3D-printed FIREFLY pedicle screw navigation system, extending its use into the European market. The technology is already available in Australia and New Zealand, and has been approved for...
Aakash Agarwal (Department of Bioengineering and Orthopaedic Surgery, Engineering Center for Orthopaedic Research Excellence, University of Toledo, Toledo, USA) has filed an official citizen petition to the US Food and Drug Administration (FDA) calling for a ban on the...
New results suggest that patients treated with chronic opioids prior to spine surgery are “significantly less likely” to achieve meaningful improvements at one-year in pain function and quality of life; and less likely to be satisfied at one-year with...
Researchers at the University of Toronto (Toronto, Canada) have released “exciting” proof-of-concept data that genetically-engineered SMaRT cells can degrade CSPGs in vitro and that human neural stem cell (NSC) grafts can form long axonal processes in the chronic cervical...
For the first time, researchers at University of California San Diego School of Medicine and Institute of Engineering in Medicine (La Jolla, USA) have used rapid 3D printing technologies to create a spinal cord, then successfully implanted that scaffolding,...
A successful first-in-human surgical procedure utilising ARAI, an augmented reality and artificial intelligence based surgical navigation system (HoloSurgical) has been carried out.
The ARAI is an advanced digital surgery platform that combines 3D visualisation, data analytics, and machine learning to...
Ulrich medical USA has announced the market entry of a vertebral body replacement device which is the company's flagship technology in the US spine implant market.
The Solidity Vertebral Body Replacement (VBR) device recently received FDA clearance and the world's first implantation of...
The Japanese Ministry of Health, Labor and Welfare has granted a marketing authorisation for EVENITY (romosozumab; Amgen and UCB) for the treatment of osteoporosis in patients at high risk of fracture.
Amgen and UCB are co-developing EVENITY worldwide, with development...
SpineSource has acquired the intellectual property assets of two spinal implant systems from Kisco International (France).
The acquisition includes the L-VARLOCK expandable lumbar cage which SpineSource has marketed, sold and distributed in the United States since 2016. The acquisition also...
It was announced at the JPMorgan Healthcare Conference (7–10 January, San Francisco, USA) that SpinalCyte has been issued a new Australian patent related to its fibroblast technology. The company’s portfolio in spine treatments now includes 36 US and foreign patents...
It has been announced that IDCT (DiscGenics) has passed the initial planned safety review of its Phase I/II trial evaluating the allogeneic, injectable disc cell therapy in patients with mild to moderate degenerative disc disease (DDD).
In this first planned...
NuVasive today announced a new organisational structure and associated executive team that chief executive officer J Christopher Barry has selected. Barry joined the company as CEO on 5 November, 2018.
As part of the new organisational structure, NuVasive announced the following...
Spineway, in collaboration with its Peruvian distributor, presented its operating techniques to some one hundred local surgeons during a recent symposium in Peru.
Established in Peru since 2014 thanks to the leading distributor in orthopaedic surgery and neurosurgery, Spineway decided...
The Society for Brain Mapping and Therapeutics (SBMT) and the Brain Mapping Foundation (BMF) recently hosted their 5th Annual Neuroscience-20 event in Buenos Aires, Argentina (N20; 26–27 November). For the past five years the goal has been to get...
Swedish digital healthcare provider, Min Doktor, and Kaia Health, a digital therapeutics company, have partnered to offer back pain treatment through a mobile application. A recent study found that Kaia Health’s artificial intelligence (AI) app reduced user-reported back pain...
Medtronic today announced it has completed the acquisition of Mazor Robotics. The total value of the transaction is reported at US$1.7 billion, or US$1.3 billion net of Medtronic's existing stake in Mazor and cash acquired.
Under the terms of the acquisition...
The RESPONSE 4.5/5.0mm System (OrthoPediatrics) has been launched in the USA. The company’s newest system, which received US Food and Drug Administration (FDA) 510(k) clearance in October, represents a product expansion for physicians to treat complex scoliosis in smaller...
The ExcelsiusGPS robotic guidance and navigation system (Globus Medical) has been installed in several hospitals across Europe.
The system won CE Mark clearance in the EU in early 2017 with indications for use in both minimally invasive and open procedures for orthopaedics...
The Monolith corpectomy system (NuVasive) has been granted expanded FDA 510(k) clearance.
The system is now cleared for procedures in the cervical spine, between the C3-C7 vertebral bodies, to treat diseased or damaged vertebral body caused by fractures, tumours, osteomyelitis...
The Japanese Pharmaceuticals and Medical Devices Agency (PMDA) has approved a Clinical Trial Notification (CTN) application for IDCT (DiscGenics), an allogenic injectable disc cell therapy for the treatment of degenerative disc disease (DDD).
IDCT is available off-the-shelf and offers a...
A Select Health of South Carolina clinical policy, dated 1 September, 2018, has been issued for the exclusive coverage of the coflex interlaminar stabilisation device (Paradigm Spine) for the treatment of lumbar spinal stenosis in patients meeting certain eligibility...
Based on its recent analysis of the North America spinal fusion device performance enhancers market, Frost & Sullivan recognises Vallum with the 2018 North America New Product Innovation Award for its interbody spinal fusion device, PEEKplus.
“To enable bioactivity and initiate...
The successful start of a first in human clinical study for the zLOCK spinal facet joint fixation system (ZygoFix) has been announced. The clinical study comprised several procedures to date and a six-month follow-up of the first case.
The first...
At the 2018 annual meeting of the German Spine Society (DWG; 6—8 December, Wiesbaden, Germany), joimax will be introducing its Endoscopic Generation 4 Devices and showcasing new 3D-printed titanium implants.
This year, joimax will be focusing on their Endoscopic Tower Generation...
Bob Paulson and Phil Soran have been appointed to Spineology’s board of directors.
Phil Soran is an entrepreneur who has founded several technology companies and was inducted into the Minnesota Business Hall of Fame in 2016. He was co-founder, president,...
FDA 510(k) clearance has been granted for the SAXXONY posterior cervical thoracic system (Nexxt Spine). The system is designed to stabilise cervical (C1 to C7) and thoracic (T1 to T3) spinal segments via posterior screw fixation in patients with...
It has been announced that Mainstay Medical will participate in the upcoming 13th German Spine Congress of the Deutsche Wirbelsäulengesellschaft (DWG; 6—8 December, Wiesbaden, Germany). DWG will be the first medical meeting at which pivotal clinical data from the...
A recent study found that using a minimally invasive technique (MIS) for the placement of pedicle screws at the upper instrumented vertebra lowered the incidence of proximal junctional kyphosis (PJK) and revision surgery for PJK at two years. The...
The US Food and Drug Administration (FDA) Bone, Reproductive and Urologic Drugs Advisory Committee (BRUDAC) are to review data supporting the Biologics License Application (BLA) for Evenity (romosozumab) (Amgen and UCB) for the treatment of osteoporosis in postmenopausal women at...
FDA 510(k) clearance has been granted for the Olympic minimally invasive surgery (MIS) posterior spinal fixation system (Astura Medical).
The Olympic MIS system delivers a new level of intraoperative flexibility and efficiency by allowing surgeons to customise to their preferred...
A partnership has been announced between FundamentalVR and University College London Hospitals NHS Foundation Trust (UCLH). Two surgical simulators have been installed at UCLH’s flagship University College Hospital (London, UK).
UCLH the first in Europe to have adopted the VR...
The FDA has cleared SpinalCyte’s Investigational New Drug (IND) protocol for CybroCell. This is considered to be the first IND approval for a fibroblast cell therapy in a chronic condition outside of dermatological uses.
The clearance allows SpinalCyte to begin...
The US FDA has announced plans to modernise its 510(k) clearance programme for approving medical devices for the US market. Data show that about 20% of current 510(k) devices are approved on trials that compare novel devices to predicate...
The first US clinical human use of the Voyant system (Viseon) for minimally invasive spine surgery access, illumination and visualisation has been announced. The case was performed by neurosurgeon John J. Knightly of the Atlantic NeuroSurgical Specialists in Morristown,...
An AmeriHealth Caritas Clinical Policy, dated 1 September, 2018, has been issued for the exclusive coverage of the coflex interlaminar stabilisation device (Paradigm Spine) for the treatment of lumbar spinal stenosis.
Lumbar spinal stenosis (LSS) affects 1.6 million patients annually and...
Zimmer Biomet recalled 1,360 spinal fusion and long bone stimulators due to a lack of adequate validation and controls to ensure product cleanliness. The FDA has identified this as a Class I recall, making it the most serious type...
CT-guided pulsed radiofrequency is safe and effective in people with acute lower back pain that have not responded to conservative treatment, according to a study presented at the annual meeting of the Radiological Society of North America (RSNA; 25–30...
At this year's annual meeting of the Radiological Society of North America (RSNA; 25-30 November, Chicago, USA), Ziehm Imaging presents a range of mobile C-arms to provide suitable options for surgeons’ different individual demands. Their Vision RFD 3D and...
A self-described “long-shot” and in the first generation of her family to attend university, Donna Ohnmeiss speaks to Spinal News International about why she chose to put her degree in mathematics to use in clinical research, and how her work on...
As emerging and existing technologies continue to shape the way we communicate and spread knowledge, the potentials of digital learning is given increasing consideration. At the recent Eurospine 2018 annual meeting (19–21 September, Barcelona, Spain), the importance of remote...
“Dear Distinguished Professor, we invite you to submit...” Christopher M Bono warns of the rise in predatory publishing, drawing on his own experiences of falling for a well-worded scam email. As editor-in-chief of the North American Spine Society’s flagship...
Statera Spine has announced the formation of the company as a subsidiary of Ortho Kinematics.
Statera will leverage the operational backbone and technology platform developed by Ortho Kinematics, but will focus on the commercialisation of Profile-ESP. Profile-ESP is an analytics...
A recent study has reported significant improvements in patients’ frailty status at one-year after surgery, as shown by the cervical deformity frailty index (CD-FI). The data, including a discussion about the efficacy of the index tool itself, were presented...
Costs were found to decrease for implants in surgery when prices were known, concludes a study presented by Andrew Glennie from Dalhousie University, Halifax, Canada, at the Eurospine 2018 annual meeting (19–21 September, Barcelona, Spain).
“Suffice to say there is quite a...
Paul Arnold, a spine surgeon based in Kansas for most of his career, now practising at the University of Illinois, USA, and current chair of the ethics and professionalism committee of the North American Spine Society (NASS), talks to...
EOS imaging have announced the first two installations in Barcelona, Spain at Clavel's Instituto, a spine centre of Hospital Quiron, and the HM Delfos Hospital. In addition, the first installation in Portugal is planned in Lisbon by the end...
Mazor Robotics has announced that at a Special General Meeting of Shareholders held on 19 November, 2018, Mazor shareholders approved the previously announced definitive merger agreement with wholly-owned subsidiaries of Medtronic.
Approximately 53% of Mazor Robotics ordinary shares were represented...
SurGenTec has announced the successful completion of the initial clinical launch of its new GraftGun bone graft delivery system combined with ViBone Viable Bone Matrix prefilled tubes. This early evaluation of the combined products was focused in the Minimally Invasive...
Highlights:
Costs curbing the rise of robotics in spinal surgery
Augmented reality surgical navigation technology enables high accuracy pedicle screw placement
Eurospine audience sceptical of digital learning's potential in spinal surgery training
Scoliosis Research Society president Todd Albert’s half-day...
This advertorial has been sponsored by INVIBIO™
Jill Wright Donaldson, MD, is a neurosurgeon at Community Hospital North (Indianapolis, IN, USA), specialising in the surgical management of complex spine disorders, neoplasms of the brain and spine, and peripheral nerve entrapment....
Medicrea has announced that the 3,000th surgery utilising Medicrea’s patient-specific UNiD ASI technology has been successfully completed.
Five years after its initial launch, over 3,000 patients worldwide have benefitted from UNiD ASI, the 100% proprietary, pre-operative planning technologies and services...
Vertiflex has announced additional results from a randomised, controlled trial of its Superion Indirect Decompression System in patients with lumbar spinal stenosis (LSS). The results, published in the Journal of Pain Research, showed an 85% decrease in the proportion of...
Zimmer Biomet has announced that the US Food and Drug Administration (FDA) has approved an extension to the Mobi-C Cervical Disc labelling to include seven-year clinical results. The updated data remain consistent with the previous findings at two and...
Altus Capital Partners today announced it has acquired ChoiceSpine. Financial terms of the transaction were not disclosed.
ChoiceSpine and Knox Spine (collectively, the Company) collaborates with physicians to develop new products which incorporate current medical technology with customised patient solutions....
Baptist Medical Park Surgery Centre, an ASC facility in Pensacola, Florida, USA, announced that it has successfully completed their first SI Fusion procedure with the recently purchased Mazor Robotics Renaissance system.
“The acquisition of the Mazor Robotics Renaissance system...
RTI Surgical has announced that HealthPartners, the largest consumer governed non-profit healthcare organisation in the USA, issued a positive coverage decision for minimally invasive sacroiliac (SI) joint fusion surgery, effective 1 November, 2018.
This decision expands access to RTI’s SImmetry System...
Implanet has announced the award of CE marking for the Jazz Cap System, developed to meet the constraints of vertebral fusion indications in adults.
Jazz Cap System, which was developed principally to facilitate the treatment of degenerative conditions in adult...
Level 1 evidence, presented at EUROSPINE 2018 in Barcelona, Spain, demonstrates that i-FACTOR peptide enhanced bone graft results in significantly higher rates of fusion in uninstrumented lumbar spinal surgery than does the use of allograft.
Michael Jacobsen (Middelfart, Denmark) spoke...
The "Orthopaedic devices market size, share and trends analysis report by application (hip, knee, spine, cranio-maxillofacial, dental, SET), by product (accessories, surgical devices), and segment forecasts, 2018 - 2026" report has been added to ResearchAndMarkets.com's offering.
The global orthopaedic device market size is expected...
Aurora Spine has announced that it has acquired an exclusive licence to US patent number 9,451,986 titled “Percutaneous sacroiliac joint implant and method for surgically inserting and securing the implant into the sacroiliac joint” in an agreement with SILIF...
Biogen was announced as the winner of the Orphan Product Award at last night’s UK Prix Galien 2018, for Spinraza, which in 2017 became the first and only approved treatment for 5q spinal muscular atrophy (SMA). In addition, the...
Colin Haines and Christopher Good, spine surgeons at Virginia Spine Institute, Virginia, USA, have performed the world’s first spinal surgery using combined endoscopic and robot-guided technology. The inaugural procedure was conceived by this team of experts out of a...
NuVasive has announced the US commercial launch of Brigade Lateral, the industry's first interbody implant and instrumentation optimised for lateral anterior lumbar interbody fusion (ALIF) spine surgery.
NuVasive's Lateral ALIF is a proprietary spine procedure enabling access to L5-S1 from...
Stryker has closed its US$1.4 billion acquisition of K2M. With the acquisition, K2M will become a wholly owned subsidiary of Stryker, the companies said.
Stryker paid US$27.50 per share for each outstanding share of K2M, representing a 27% premium over K2M’s average closing...
Life Spine has announced the initiation of the PROLIFT Expandable clinical study with Ahmed Khan, Central Connecticut Neurosurgery and Spine, Connecticut, USA.
Mariusz Knap, vice president of Marketing and Business Development notes, “Clinical studies are the foundation for advancing innovative spine...
SpinalCyte has announced that a single injection of modified human dermal fibroblasts (HDFs) resulted in significant improvements of disc height and pain reduction 12 months after injection of the cell therapy for patients with degenerative disc disease (DDD).
The trial...
Simplify Medical, maker of the Simplify cervical artificial disc, has announced that it has completed the enrolment and treatment of all patients in its US Investigational Device Exemption (IDE) pivotal trial evaluating the Simplify Disc for two-level cervical disc replacement. The...
Backpack Health’s cloud-based mobile app offers paediatric scoliosis patients and their caregivers a centralised platform to manage health data and connect with the global scoliosis community.
Backpack Health and the Children’s Scoliosis Foundation (CSF) have announced a partnership that will...
Innovent Biologics, a biopharmaceutical company, has announced that the National Medical Products Administration (NMPA, successor to the CFDA) has accepted its new drug application (NDA) for adalimumab biosimilar candidate (IBI303). IBI303 is a recombinant human anti-TNF-α monoclonal antibody independently developed...
Bio2 Technologies, a privately held orthopaedics company, has announced that it received US Food and Drug Administration (FDA) approval to begin enrolment in an IDE clinical study to evaluate Vitrium as a cervical interbody fusion device. Vitrium will be evaluated...
EOS imaging has announced the change of its leadership effective 1 January 2019. The Board of Directors, in agreement with Marie Meynadier, chief executive officer of EOS imaging, has decided to change the leadership of the company to strengthen...
The era of routine robotic-assisted spinal surgery is on the horizon. Despite the hype, however, there remains little market penetration, with affordability and the degree of value-added by such technology representing significant barriers to complete disruption of standard practice.
In a recent...
RTI Surgical and Paradigm Spine have entered into a definitive agreement whereby RTI will acquire all outstanding equity interest of Paradigm Spine in a cash and stock transaction valued at up to US$300 million, consisting of US$150 million at closing plus...
A recent study found no improvements in pedicle screw accuracy but increased radiation using intraoperative computed tomography (CT)-based navigation compared to a freehand technique in idiopathic scoliosis surgery. The study was carried out by Wiktor Urbanski (Wroclaw University, Wroclaw,...
NuVasive has announced it has received 510(k) clearance from the US Food and Drug Administration (FDA) for use of its COHERE Porous PEEK implant in eXtreme Lateral Interbody Fusion (XLIF) surgical spine procedures.
NuVasive's patented Porous PEEK technology offers three-dimensional...
SpinalCyte, a regenerative medicine company focused on regrowth of the spinal disc using Human Dermal Fibroblasts (HDFs), has announced the issuance of new patents in Hong Kong and Europe. The company’s intellectual property in spine treatments now includes 35...
Spinal Elements has recently announced FDA clearance for claims related to the macro-, micro-, and nano-surface structure of its Ti-Bond surface coating technology. Utilising this advancement, the company have recently introduced the Lucent XP height- and lordosis- expandable interbody device.
Interbody...
Titan Spine, a medical device surface technology company focused on developing surface-enhanced spinal interbody fusion implants, has announced it has closed a substantial round of Series B financing with its current investment partner, Southlake Equity Group. Titan will use...
Dedicated surgical teams have been found to reduce surgical times and room times in patients with adolescent idiopathic scoliosis (AIS). Surgical times decreased by 13%, and room times by 11%, in 590 cases of AIS between 2006 and 2015....
Speaking anonymously to Spinal News International, a career and life coach for physicians said that the most effective way to address the issue of physician burnout, in his experience, is “experimental learning during the course of a three- to...
A recent study has concluded that the clinical benefits of a minimally invasive surgical technique appear to “diminish” as a function of fusion length. The data, which examined the relationship between open versus minimally invasive lumbar fusion and the...
With the introduction of new wearables designed for intraoperative use, such as the Hololens (Microsoft) head-up display, there has been considerable interest in bringing augmented reality to surgical procedures in recent years. At the North American Spine Society (NASS) annual...
TETRAfuse 3D technology (RTI Surgical) won a 2018 spine technology award from Orthopedics This Week. RTI Surgical accepted the award at the North American Spine Society’s (NASS) 33rd annual meeting (26–29 September, Los Angeles, USA).
“RTI is honoured to receive this important...
NuVasive have announced it has entered into a strategic partnership with Biedermann Technologies, a company that holds a broad and extensive patent and technology portfolio in the fields of spinal and extremity surgery, based in Donaueschingen, Germany.
Biedermann Technologies works...
Research that highlights a worrying trend between cycling and spinal trauma has recently been published in The Surgeon. Although the authors of the paper, M P Broe (Mater Misericordiae University Hospital, Dublin, Ireland) and colleagues, state that, “It is...
Rates of survival are higher, and respiratory intervention rates are lower, in 25 infants with presymptomatic 5q spinal muscular atrophy (SMA) treated with Spinraza (nusinersen). Biogen announced these new interim results from their NURTURE trial; this is an ongoing...
Medtronic has announced the US launch of the Infinity Occipitocervical-Upper Thoracic (OCT) System designed to simplify posterior cervical spine surgery. A company press release describes the Infinity OCT System as a procedural solution that integrates navigation and biologics with...
The Ennovate® PentaCore® Screw is the new benchmark in the Pedicle Screw Systems market. Ennovate screws have a significantly higher pullout force, shear strength, and overall endurance than other screws currently available on the market, concludes a recently published study...
The global spinal implants market is forecast to grow at a 7% compound annual growth rate to exceed US$19.5 billion by 2024, according to a recent Market Research Engine report.
The growth of bioresorbable implants is expected to propel the spinal implants...
Medtronic and Mazor Robotics have announced that the two companies have entered into a definitive merger agreement under which Medtronic will acquire all outstanding ordinary shares of Mazor for US$58.50 per American Depository Share, or US$29.25 (104.80 ILS) per...
ImmersiveTouch, a Chicago-based company, announced the launch of ImmersiveView, the only suite of integrated virtual reality real-time solutions for personalised surgical planning, patient engagement, and surgical training using patented haptic technology. ImmersiveView was launched at the North American Spine...
Philip Sell (Leicester and Nottingham, UK) tells Spinal News International that as the field of spine surgery evolves from using generic to more specific outcome measures, he would be delighted to see a move away from surrogate outcome measures...
Factors characterising an indication for surgery in lumbar spinal stenosis
Outcomes following surgery for spinal stenosis present great variability, suggesting that patient selection could be improved. Anne Mannion (Senior Research Fellow, Spine Centre, Schulthess Klinik, Zürich, Switzerland) tells Spinal News...
“Outcomes are important for patients. It is very important that we understand what happens to our patients when we carry out treatments, be they surgical interventions or conservative treatment. If we do not know that, we cannot adapt our...
Frank Kandziora (Frankfurt, Germany), the 2018 president of EUROSPINE, the Spine Society of Europe, tells Spinal News International that assuring quality in spine surgery is a cornerstone of patient care.
A recent study reports that new technology utilising augmented reality surgical navigation can be clinically used to place pedicle screws, enabling both high accuracy and an acceptable navigation time. The results were presented by Gustav Burström, Karolinska Institutet, Stockholm,...
Johnson & Johnson Medical Devices Companies have announced that DePuy Synthes is introducing SENTIO MMG, a first-of-its-kind digital mechanomyography platform designed to assess nerve status and identify and avoid peripheral nerves during spine surgery. SENTIO MMG enables motor nerve...
CoreLink have announced the expanded commercial launch for the FLXfit15 articulating-expandable intervertebral body fusion device.
FLXfit15 is a posterior lumbar expandable interbody device that offers up to 15 degrees of controlled and continuous expansion and is one of the only...
The Therapeutic Goods Administration (TGA) has granted approval to Nevro for its next-generation Senza II spinal cord stimulation system delivering the company’s proprietary HF10 therapy. According to Nevro, the Senza II system offers the outcomes and clinical advantages of...
Highlights:
Surgeons call for ban on pedicle screw reuse and demand two-step asepsis process
Second scoliosis surgery of the day as safe and effective as first
Many surgeons do not use patient-reported outcome measures in spine care
Scoliosis Research...
Johnson & Johnson has announced its acquisition of spinal fusion implant developer Emerging Implant Technologies for an undisclosed amount.
Norderstedt, Germany-based EIT produces 3D-printed titanium interbody implants specifically for spinal fusion surgery. The company’s devices use proprietary cellular titanium which...
Benvenue Medical, a developer of minimally invasive expandable implant solutions for lumbar fusion, has announced it has completed the divestiture of its vertebral augmentation systems portfolio for an undisclosed amount to IZI Medical Products. A company press release states...
Contaminated pedicle screws cost hundreds of thousands of US dollars a year, and through an increase in surgical site infections result in additional and avoidable patient morbidity. Anand Agarwal (University of Toledo, Toledo, USA) and colleagues are calling for...
Once an aspiring architect, Frank Kandziora has never looked back from the medical profession since being assigned a medical assistant during his military service in Germany. As the outgoing president of EUROSPINE, he reflects on the successes of the...
Invuity, a medical technology company focused on advanced surgical devices to enable better visualisation, announced that it has entered into a definitive agreement with Stryker, pursuant to which Stryker will acquire all of the outstanding shares of Invuity...
New research outlines how spinal injuries in suicidal jumpers differ from those in non-suicidal patients with regard to patient demographics, mental health condition, injury location, neurological damage and associated injuries. The study was carried out by Hiroki Kano and...
Paradigm Spine, a company specialising in the treatment of lumbar spinal stenosis, has announced the issuance of a broad coverage medical policy from BlueCross BlueShield of South Carolina covering its coflex device for the surgical treatment of lumbar spinal...
Captiva Spine has announced it has received 510(k) clearance from the US Food and Drug Administration (FDA) to market its TirboLOX-L 3D printed Titanium lumbar cages.
TirboLOX-L titanium lumbar cages are created using advanced 3D printing technologies to form titanium...
The Spine Center at Dignity Health St. Mary's Medical Center (San Francisco, USA), has been selected to participate in a FDA/IDE pivotal study, sponsored by Premia Spine, studying the use of the Tops System. St. Mary's Medical Center is...
Todd H Lanman is a spine surgeon known for his work on the advancement of total disc replacement surgery and motion preservation. Following his own experiences undergoing a number of spinal surgeries, Lanman has pursued a career dedicated to the restoration of his...
EOS imaging, a 2D/3D imaging company for orthopaedics, has announced the first installation of an EOS system in Mexico, the largest Central American market, at Shriners Hospitals for Children—Mexico, located in Mexico City.
“Shriners Hospitals for Children—Mexico, noted for excellent...
UK’s National Institute for Health and Care Excellence (NICE) has published its Appraisal Consultation Document (ACD) outlining a ‘minded no’ for the routine funding of Spinraza (nusinersen) for the treatment of 5q spinal muscular atrophy (SMA) in England, Wales...
Eden Spine’s Sphynx plating system has been granted FDA clearance, the company has announced.
Sphynx was designed to complement the company’s Giza titanium vertebral body replacement with rotatable endplates, introduced in 2012. The Giza is intended to replace and fuse a...
SI-Bone has announced that iFuse will be added to the List of Refundable Products and Services in France (Liste des Produits et Prestations Remboursables- LPPR), meaning that the French National Healthcare System will exclusively cover the iFuse procedure. Through...
Todd J Albert is surgeon-in-chief at the Hospital for Special Surgery, New York, USA, and is the current president of the Scoliosis Research Society (SRS). At the 25th International Meeting on Advanced Spine Techniques (IMAST; 11–14 July, Los Angeles,...
NuVasive has announced the Pulse surgical automation platform has received 510(k) clearance from the US Food and Drug Administration (FDA). Pulse is the foundation for the company’s Surgical Intelligence system, and introduces 2D- and 3D-navigation and smart imaging capabilities...
Independence in mobility is the single most important factor affecting quality of life in patients with traumatic spinal cord injury, reports a study in the American Journal of Physical Medicine & Rehabilitation, the official journal of the Association of Academic Physiatrists.
Based on validated clinical questionnaires, the study by Julien...
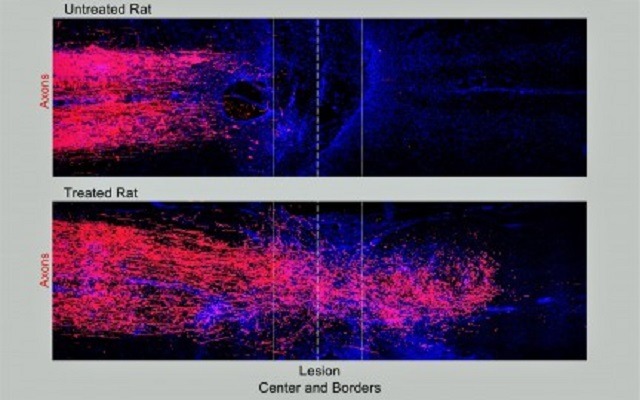
Neuroscientists at UCLA, Harvard University and the Swiss Federal Institute of Technology have identified a three-pronged treatment that triggers axons to regrow after complete spinal cord injury in rodents. In addition to facilitating axon growth through scar tissue, the...
Nusinersen (Biogen) has previously been shown to be effective in the treatment of spinal muscular atrophy in infants under seven months of age; recent research published in Neurology, the medical journal of the American Academy of Neurology, suggests that...
Stryker has announced a definitive merger agreement to acquire all of the issued and outstanding shares of common stock of K2M Group Holdings, a company specialising in minimally invasive spinal devices, for US$27.50 per share, or total equity value...
A novel approach reported by Peter Pijpker and colleagues from the University Medical Center Groningen, Groningen, The Netherlands, outlines the use of 3D virtual planning and 3D-printed models that have the ability to change how complex spinal surgeries are...
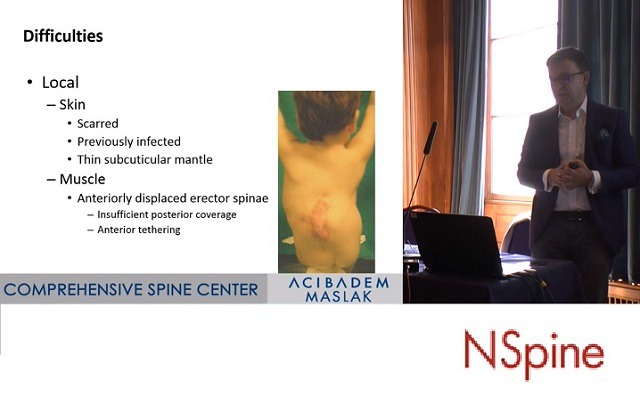
At the NSpine meeting (London, UK), Ahmet Alanay (Istanbul, Turkey) discussed the surgical management of deformity in spina bifida patients. You can watch the full talk and subsequent discussion below.
According to Woojin Cho (Montefiore Medical Center, the University Hospital for Albert Einstein College of Medicine, New York, USA), who presented at the 25th International Meeting on Advanced Spine Techniques (IMAST; July 11-14, Los Angeles, USA), spinal surgeons continue to wrongly recommend...
NuVasive and Siemens Healthineers have announced a strategic partnership, which is to focus on “technology development, marketing and commercial activities to advance clinical outcomes in minimally invasive spine surgery”, according to the two companies.
NuVasive is a spine health technology...
Izana Bioscience, a biopharmaceutical company focused on translational medicine, has announced the initiation of a phase II proof-of-concept clinical study of namilumab in ankylosing spondylitis, a debilitating arthritic disease of the spine that affects millions of people worldwide.
The randomised,...
Scoliosis correction is an extensive surgery, and operating on multiple patients in one day can be exhausting for the surgical team. However, recent research finds that the second surgery of the day has similar outcomes and complication rates compared...
Following the completion of its first surgical cases, K2M has received Food and Drug Administration (FDA) 510(k) clearance and a CE mark for its Cayman United Plate System.
The Cayman United Plate System is designed for rigid fixation to K2M's...
At the NSpine meeting (London, UK), Alessandro Gasbarrini (Bologna, Italy) discussed decision making in recurrent spinal tumours. Click to watch the full talk here.
Connecticut Orthopaedic Specialists have announced the enrollment of the initial patients within Simplify Medical’s clinical trial to evaluate the Simplify Disc, its novel investigational cervical disc, at two adjacent cervical levels. James J Yue, a spine and neck specialist...
Bioventus has entered into a definitive agreement to divest its next-generation bone morphogenetic protein (BMP) development programme to a new company formed by Viscogliosi Brothers, a private equity investment firm focused on developing innovative neuromusculoskeletal technologies.
Bioventus acquired the exclusive,...
The first patients have been enrolled in an investigational device exemption (IDE) clinical trial evaluating the safety and efficacy of the next-generation P-15L peptide enhanced bone graft (Cerapedics) in transforaminal lumbar interbody fusion (TLIF) surgery. Cerapedics gained FDA approval...
A novel immune-evasive gene therapy has restored skilled grasping function in paralysed rats, allowing treated rodents to independently reach for and pick up sugar cubes, research recently published in Brain reports. The findings of the preclinical study support the...
Concorde Lift (DePuy Synthes), a new expandable interbody device, is launching in the USA. The implant is designed to treat patients suffering from degenerative disc disease as part of the new offering called UNLEASH MIS TLIF (Transforaminal Lumbar Interbody...
At the NSpine meeting (London, UK), Bronek Boszczyk (Benedictus Clinic Tutzing, Germany) discussed combined convex and concave costoplasty for posterior chest wall in adolescent idiopathic scoliosis (AIS).
In Lenke type 1 or type 2 adolescent idiopathic scoliosis (AIS), significant correction of the main thoracic curve with relative under-correction of the proximal thoracic curve increases the incidence of postoperative shoulder height imbalance. This is the conclusion presented...
The next evolution of the Reline system (NuVasive) has just launched: the Reline MAS Midline (RMM). The company description says this system provides “procedural versatility in a compact midline construct”.
RMM incorporates low-profile modular implants and advanced system instrumentation to...
The US Food and Drug Administration (FDA) has granted approval to market a polyetheretherketone (PEEK) spinal interbody fusion device with a PEEKplus nanotextured surface (Vallum Corporation). The PEEKplus nanotextured surface is the first and only FDA-cleared nanotextured surface on...
Cerapedics has announced that the company completed a US$22 million financing led by KCK Group, a family investment fund that focuses on innovative medical technologies that meet significant clinical needs.
This equity funding will accelerate the commercial release of i-FACTOR...
The results of a report looking at the rates of spinopelvic malalignment in nearly 600 patients show that malalignment is common both before and after short-segment degenerative fusions. These findings are detailed in the first peer-reviewed publication from the...
Mainstay Medical International, a medical device company focused on bringing to market ReActiv8, an implantable neurostimulation system to treat chronic low back pain, announces the completion of all implants in ReActiv8-B, its US IDE clinical study.
A total of 204...
Spine surgeons earn high ratings for their skill and good clinical outcomes on internet review sites—but are more likely to receive negative ratings and comments on factors pertaining to clinic staff, billing, and wait times, reports a landmark study...
EOS imaging has announced the installation of its EOS system at the University Hospital Center of Grenoble Alps (CHUGA), a state-of-the-art hospital facility for osteoarticular surgery in children and adults. CHUGA is the 20th university hospital centre to acquire an...
HD LifeSciences has received FDA 510(k) clearance for its Hive-C IBFD, a system of interbody devices for anterior cervical fusion procedures.
The Hive-C implant system will be commercially available in July 2018.
The devices are based on the company’s patented, additive-manufactured Soft Titanium...
South Australian researchers are embarking on a AUD$20 million medical and manufacturing research project which could reduce the chance of infection after orthopaedic surgery, thanks to a little help from the humble dragonfly.
Working with leading surgeons and an Australian...
The first surgeries with robotically assisted minimally disruptive placement of the Centerline cortical screw system and the Prolift expandable spacer system (Life Spine) in an ambulatory surgical centre have been announced.
“Life Spine is dedicated to address and support minimally...
A recent study, initially published in Nature Communications, shows that anti-oxidation specific epitope (OSE) antibodies protect against osteoporosis, illuminating the potential for a novel approach to treatment.
The data presented in the report by Elena Ambrogini (Division of Endocrinology, Department...
The use of i-FACTOR peptide enhanced bone graft (Cerapedics) resulted in a 50% fusion rate, compared to a 20% fusion rate using allograft in patients with non-instrumented surgery. This is the result of the IVANOS study evaluating i-FACTOR peptide...
Renovis Surgical Technologies has announced that it has received 510(k) clearance from the US Food and Drug Administration (FDA) to market the Tesera SA Hyperlordotic ALIF interbody spinal fusion system.
Tesera SA is a porous titanium stand-alone anterior lumbar interbody...
The results of the COAST-W trial demonstrate that Taltz (ixekizumab; Eli Lilly and Company) is a safe and effective treatment for Ankylosing Spondylitis (AS), also known as radiographic axial spondyloarthritis. COAST-W is a Phase 3 trial, and is the...
EOS imaging has announced the installation of an EOS imaging system at ATOS Klinik Heidelberg, establishing it as the first private practice in Germany to offer the low-dose 2D/3D imaging system.
The system will be available at the spinal surgery...
As a multidisciplinary membership organization, the North American Spine Society (NASS) has an incredible group of volunteers who work on many initiatives in the research, health policy, education, communications and advocacy arenas. This supplement will provide an update on...
Paediatric patients with severe spinal deformity are at a high risk of revision surgeries. Presenting his research at the Global Spine Congress (GSC; 2–5 May, Singapore), Munish Gupta (Department of Orthopedics, Washington University, St. Louis, USA) reported a 12%...
The A-CIFT SoloFuse HA (SpineFrontier) has received FDA approval.
The company states that the A-CIFT SoloFuse HA standalone system was produced “to leverage the familiarity of existing techniques, while providing an alternative to traditional plating for one level procedures—with the...
The annual census of UK consultants and higher speciality trainees—Focus on Physicians 2017–18—indicates that more than half of all consultants and two thirds of trainees reported frequent gaps in trainees’ rotas, with one in five respondents saying these are...
Back Pain Centers of America, a call centre which connects people searching for solutions to their neck and back pain with spine specialists, have released a spine health e-book entitled Critical Factors for Successful Spine Surgery. This 65-page free...
US spine care providers have a brand new tool to measure and improve patient care: a diagnosis-based clinical data registry that tracks patient care and outcomes. Launched by the North American Spine Society (NASS), this web-based platform will allow...
The US Food and Drug Administration (FDA) have granted clearance for the Catamaran sacroiliac joint fixation system (Tenon Medical) specifically indicated for sacroiliac joint fusion for conditions including sacroiliac joint disruptions and degenerative sacroilitis.
The Catamaran sacroiliac joint fixation system...
The US Food and Drug Administration (FDA) has granted 510(k) clearance of Kyphon HV-R bone cement (Medtronic) for fixation of pathological fractures of the sacral vertebral body (or ala) using sacral vertebroplasty or sacroplasty.
Medtronic state that this broadens the...
Porous polyetheretherketone (PEEK) technology (NuVasive) is a clinically viable alternative for improving osseointegration and fusion rates of interbody implants to treat degenerative cervical disc disease, a recent paper in the Journal of Spine & Neurosurgery reports.
In the study, 50...
“Decompression and fusion for degenerative spondylolisthesis is associated with reduced risk of opioid dependency”, says Mayur Sharma (Department of Neurosurgery, University of Louisville, Louisville, USA), summarising his latest research, recently published in the Journal of Neurosurgery: Spine. The study...
TriStar Centennial Medical Center (Nashville, USA) is the first healthcare system to incorporate the Levó head positioning system (Mizuho OSI) into their operating room. Mizuho OSI state claim that the system confers better control and safety of a patient’s...
EOS imaging, a 2D/3D imaging and data solutions for orthopaedics, announced the launch of EOSone, its new private practice programme, at Becker’s 16th Annual Future of Spine + The Spine, Orthopedic and Pain Management Driven ASC Conference (14–16 June,...
The latest line of icotec interbody cages, designed to optimise bony integration and post-operative visualisation, has received US Food and Drug Administration (FDA) 510(k) clearance. The clearance includes cages for a variety of surgical approaches, such as cervical fusion...
Almost one third of spine surgeons do not routinely use patient-reported outcome measures (PROMs). This was the result presented by Asdrubal Falavigna (Department of Neurosurgery, Caxias do Sul University, Caxias do Sul, Brazil) at the Global Spine Congress...
A preliminary study has demonstrated the feasibility of using a novel visualisation approach as a valuable adjunct tool for minimally invasive percutaneous procedures. This is the conclusion of Gerard Deib (Division of Interventional Neuroradiology, The Johns Hopkins Hospital, Baltimore,...
The use of a hydrogel after spinal cord injury is responsible for the restoration of independent breathing control in rats, according to research recently published in The Journal of Neuroscience.
Researchers at Jefferson, Angelo Lepore (Department of Neuroscience, Philadelphia University and Thomas...
The commercial launch of Fortilink-TS and –L IBF systems (RTI Surgical) with TETRAfuse 3D technology has added to a growing series of interbody fusion devices featuring RTI Surgical’s proprietary TETRAfuse 3D technology.
The Fortilink-TS and -L systems are intended for...
Three factors were found to be predictive of survival in a surgical series of metastatic epidural spinal cord compression, including the type of primary tumor and a lower degree of physical disability on the SF-36 physical component score. These...
Patients who take prescription opioids for a longer period before spinal surgery are more likely to continue opioid use several months after surgery, reports a study in The Journal of Bone & Joint Surgery.
According to the new research, led by Andrew...
Amgen has announced that the European Commission (EC) has approved a new indication for Prolia (denosumab) for the treatment of bone loss associated with long-term systemic glucocorticoid therapy in adult patients at increased risk of fracture. The EC approval is based on...
CoreLink, a manufacturer of spinal implant systems, acquired Israel-based Expanding Orthopedics, a privately held medical device developer.
Along with the FDA cleared expanding and articulating FLXfit and FLXfit 15 titanium TLIF interbody systems, CoreLink has also acquired a broad array...
According to a study published in BMJ Open, just over half of patients think what a doctor wears is important and more than a third claim that their doctor’s attire influences the satisfaction they have with the care that...
The North American Spine Society (NASS) has issued a coverage policy recommendation for Lumbar Interlaminar Device without Fusion and with Decompression, which applies to Paradigm Spine’s coflex device. This recommendation is particularly significant for coflex because it provides the...
The global spine surgery products market is expected to reach US$16.7 billion by 2025, up from US$10.2 billion in 2016, a Transparency Market Research report states. The report anticipates that the spine surgery products market will grow at a...
The US Food and Drug Administration (FDA) has granted pre-market supplemental approval (PMA) for the coflex interlaminar stabilisation disposable instrument kit (Paradigm Spine). This marks the first approved disposable instrument set for a Class III spinal device to receive...
CIMZIA (certolizumab pegol) is the first therapy to demonstrate positive results in a 52-week, placebo controlled non-radiographic axial spondyloarthritis study. The positive topline results from C-AXSPAND, a Phase 3 multi-centre, randomised, double-blind, parallel-group placebo controlled study to investigate the...
Countries in the European Union have long been the first to receive new innovations in medical technology, as the EU’s Medical Device Directive (MDD) provided quicker routes to implementation of new devices than its equivalent in the USA, the...
An experimental drug that blocks abnormal neural communication after spinal cord injury could one day be the key to improving quality of life by improving bladder function, new research published in The Journal of Clinical Investigation suggests.
Researchers at The...
The Rampart One Standard anterior lumbar interbody fusion (ALIF) device (Spineology) has been granted FDA clearance, allowing it to be used with or without supplemental fixation.
The Rampart One ALIF interbody fusion system is an anatomy-conserving technology, a press release...
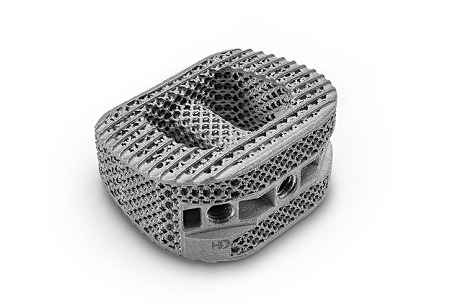
The US Food and Drug Administration (FDA) has granted 510(k) clearance for both the ALTA ACDF interbody spacers (Astura Medical) and HALF DOME lumbar interbody spacers in PEEK-OPTIMA Hydroxyapatite Enhanced (Invibio Biomaterial Solutions).
PEEK-OPTIMA HA Enhanced provides an innovative biomaterial...
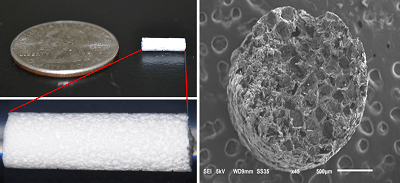
The use of a bone-anchored anular closure device following discectomy is superior to treating high-risk patients with discectomy alone, a two-year study recently published in The Spine Journal reports. The landmark, randomised superiority clinical trial investigated the Barricaid anular...
The US Food and Drug Administration (FDA) has given 510(k) clearance to Camber Spine to market its ENZA-A titanium anterior lumbar interbody fusion (ALIF) system, a unique, minimally invasive interbody fusion device providing integrated fixation.
ENZA-A Titanium ALIF is an...
The committee for medicinal products for human use (CHMP) of the European Medicines Agency (EMA) has adopted a positive opinion in the marketing authorisation of Prolia (denosumab) for the treatment of bone loss associated with long-term systemic glucocorticoid therapy...
Intraspinal injections of human spinal cord-derived neural stem cells are well tolerated in a Phase I study of four patients with thoracic (T2–T12) ASIA-A grade spinal cord injury. This was the result recently published in Cell Stem Cell, supporting...
Researchers at Hospital for Special Surgery (New York, USA) have launched a pilot study to see how a rheumatologic agent that treats several related autoimmune disorders affects the skeleton.
The two-year study, led by Susan M Goodman (Hospital for Spinal Surgery, New...
A Loyola Medicine study has found that 15.4% of patients who take drug holidays from osteoporosis drugs called bisphosphonates experienced bone fractures. During a six-year follow-up period, the yearly incidence of fractures ranged from 3.7% to 9.9%, with the most fractures...
Case performed by Oded Hershkovich and Bronek Boszczyk (both Centre for Spinal Studies and Surgery, Queen’s Medical Centre, Nottingham, UK). nspine
Introduction
We would like to present an interesting clinical case of adolescent idiopathic scoliosis (AIS), in which the concave costoplasty...
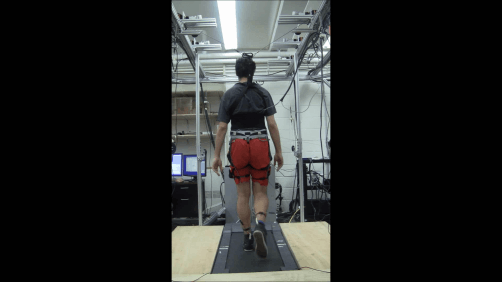
The world’s first advanced robotic treatment device that has been evidenced to improve a patient’s ability to walk is being made commercially available in the USA. Individuals with spinal cord injuries can now access FDA-cleared HAL, which is short...
Massachusetts General Hospital surgeons have proposed the development of a screening measurement to determine the likelihood of prospective National Football League (NFL) players suffering a potentially career-ending degree of cervical stenosis. This research was presented by Mark Callanan (Massachusetts General Hospital...
Spinal stimulation increases the odds of pain relief more than medical therapy when patients are faced with intractable spine or limb pain. This is the result of a recent study, presented in a scientific poster at the American Academy...
Spinal anaesthesia is safe for high-risk patients undergoing lumbar spinal surgery, and allows for better perioperative hemodynamic stability compared to general anesthesia, a recent study published in the Journal of Clinical Anesthesia reports.
The study, by Michael Finsterwald (Department of Orthopedic Surgery, Balgrist...
A preliminary study suggests that an investigational drug may help increase protein levels in infants with spinal muscular atrophy. The results of the open label study were presented at the American Academy of Neurology’s 70th Annual Meeting (21–27 April, Los Angeles, USA).
Spinal muscular atrophy...
Patients who have been taking opioids as pain relievers for several months before spinal fusion surgery are at increased risk of complications after their surgery, reports a study in Spine. The results were presented at the 18th annual conference...
Surgeons’ procedural choices and patient malalignment are the strongest predictors of distal junctional kyphosis in the first year following surgery. This was the conclusion presented by Peter Passias (Department of Orthopaedic Surgery, NYU Langone Orthopedic Hospital, New York, USA)...
Percutaneous endoscopic lumbar discectomy via an interlaminar approach is effective for lumbar disc herniation, with few complications, reported Jiancheng Zeng (West China Hospital, Sichuan University, Department of Orthopaedics, Chengdu, China), speaking at the 18th annual conference of the International...
Martin Underwood (Warwick Medical School, University of Warwick, Coventry, UK) has worked as a general practioner in Lusaka, Manchester, London and Coventry, UK. He has a track record of community based research into the improved diagnosis and management of musculoskeletal disorders, particularly back...
An advocate for interprofessional models of care for spine and musculoskeletal disorders, Raja Rampersaud is a spine surgeon globally recognised as a leader and innovator in the minimally invasive field. His clinical research focuses on health services and quality of...
Postoperative gait disturbance significantly increases with age, persisting in elderly patients for longer than in non-elderly patients. This is the finding of a recent study comparing clinical and radiographic outcomes between elderly and non-elderly patients with cervical spondylotic myelopathy undergoing laminoplasty. The...
EOS imaging has announced that University Orthopedics in Providence, Rhode Island, and the Hey Clinic for Scoliosis and Spine Surgery in Raleigh, North Carolina (both USA) have installed the EOS system for low-dose, 2D and 3D imaging of patients. Both...
Both bracing and exercise have demonstrable, significant treatment effectiveness for patients with adolescent idiopathic scoliosis. This was the finding of a randomised, controlled trial presenting level one evidence, carried out by Yu Zheng (Interdisciplinary Division of Biomedical Engineering, The...
The US Food and Drug Administration (FDA) has given 510(k) clearance for the Zyston strut open titanium interbody spacer system (Zimmer Biomet). This marks Zimmer Biomet’s first titanium spinal implant manufactured via a 3D printing process.
The Zyston strut open...
Both free hand and robotic-guided S2 alar-iliac (S2AI) screw placement prove to be safe, accurate and reliable techniques for achieving spinopelvic fixation. This is the finding of Jamal Shillingford (Department of Orthopaedic Surgery, Columbia University Medical Center, The Spine...
The international Society on Scoliosis Orthopaedic Rehabilitation and Treatment (SOSORT) elected Luke Stikeleather as president at its annual meeting held this spring in Dubrovnik, Croatia. Stikeleather is the founder and president of the National Scoliosis Center located in Fairfax (USA).
SOSORT...
The US Food and Drug Administration (FDA) has given 510(k) clearance to the PRO-LINK titanium stand-alone cervical spacer system (Life Spine) for spinal fusions.
PRO-LINK feature’s Life Spine’s OSSEO-LOC surface treatment, and has experienced great success since inception, a press...
A novel endoscopic, stand-alone transforaminal decompression and fusion (TLIF) technique using the VariLift-LX system (Wenzel Spine) has been described in a 24 consecutive patient case series, published in the Journal of Spine.
The VariLift-LX system is a stand-alone, threaded expandable...
Prominent spinal neurosurgeon Todd H Lanman (Lanman Spinal Neurosurgery, Los Angeles, USA) has been honored by the Los Angeles Business Journal as one of its 2018 ‘Leaders in Health Care,’ taking home the prestigious award at the 2018 Health...
Using stem cell derived neuronal cells reprogrammed from genetically identical animals eliminated the need for immunosuppression measures.
A major hurdle to using neural stem cells derived from genetically different donors to replace damaged or destroyed tissues, such as in a...
Bruce Dall is a spine surgeon, having gained his medical qualification at the University of Nebraska Medical School (Omaha, USA), and an advocate for those with sacroiliac joint pain, publishing multiple papers on the joint. Dall edited the textbook Surgery...
Recovery after severe spinal cord injury is notoriously fraught, with permanent paralysis often the result. In recent years, researchers have increasingly turned to stem cell-based therapies as a potential method for repairing and replacing damaged nerve cells. They have...
Spinal Elements, a spine technology company, has announced the release of its Clutch interspinous process device. This new product further enhances the breadth of Spinal Elements’ thoracolumbar portfolio and offers surgeons more options for treatment of various posterior thoracolumbar...
Percutaneous vertebroplasty does not result in statistically significant greater pain relief than a sham procedure, reports a study detailing the results of the VERTOS IV trial, published in the British Medical Journal (BMJ). This study followed patients with acute...
Michael Jackson's gravity-defying Smooth Criminal dance move, which wowed live audiences and inspired new forms of dancing, was down to core strength and an illusion, neurosurgeons write in The Journal of Neurosurgery: Spine.
Dubbed the anti-gravity tilt, the seemingly impossible...
In a small cohort of patients, spinal cord stimulation has been shown to restore volitional movement in select patients with paraplegia, after intensive therapy. This was the result presented by winner of the Philip L Gildenberg Resident Award, David...
A change in policy allowing overlapping surgery decreases length of stay in an academic, safety net hospital. This is the conclusion of Anthony DiGiorgio (Department of Neurosurgery, Louisiana State University Health Sciences Center, New Orleans; Department of Neurosurgery, University...
Researchers trying to help people suffering from paralysis after a spinal cord injury or stroke mapped critical brain-to-spinal cord nerve connections that drive voluntary movement in forelimbs, a development that scientists say allows them to start looking for specific...
A new study led by Johns Hopkins researchers adds to growing evidence that patients underuse non-opioid pain relievers to supplement opioid pain management after spine and joint surgery.
A report on the findings, which also shows that patients improperly store and dispose...
DiscGenics, a clinical stage regenerative medicine company, has announced the first patient has been treated in its phase I/II US clinical trial of IDCT for mild-to-moderate degenerative disc disease. The treatment took place at Carolina Neurosurgery and Spine Associates...
Infuse Bone Graft (Medtronic) has gained US Food and Drug Administration (FDA) approval in new spine surgery indications. InfuseBone Graft is now approved for use with additional spinal implants made of polyetheretherketone (PEEK) in oblique lateral interbody fusion (OLIF...
Auto-registration is better than point-to-point registration with respect to clinical accuracy when using the same active infrared navigation system during spinal surgery. This is the conclusion of the first study to compare the difference between the two methods with...
Highlights:
Percutaneous vertebroplasty does not result in statistically significant greater pain relief than a sham procedure, according to the most recent results of the VERTOS IV trial
Surgeons’ procedural choices have strong influence on risk of distal junctional kyphosis
...
The Scottish Medicines Consortium (SMC) has recommended the routine funding of Spinraza (nusinersen) for the treatment of symptomatic type 1 5q spinal muscular atrophy (SMA) (infantile onset). The acceptance comes after the SMC took just four months to review...
EOS imaging, a company specialising in 2D/3D imaging for orthopaedics, has announced the installation of an EOS system at the Queensland X-Ray radiology practice in the Gold Coast Private Hospital in Southport, Gold Coast, Australia. The system placement is...
A recent study detailing the cellular mechanism powering the neuronal regenerative capacities of axolotls (otherwise known as Mexican salamanders) hopes to find new leads in the treatment of spinal cord injury. Lead researcher Karen Echeverri (Department of genetics, cell...
Wearable robotics do not eliminate stress—they just shift it to other parts of the body. This is the finding of a study in which researchers tested a commercially available exoskeleton—a Steadicam vest with an articulation tool support arm—typically worn...
Highlights:
New study offers hope for recovered motor function in patients with chronic spinal cord injury
New algorithm decodes spine oncology treatment
Experimental therapy restores nerve insulation damaged by autoimmune disease
Minimally invasive TLIF procedure results in shorter hospital...
Stryker’s 3D-printed Tritanium In-Growth Technology continues to impact spinal surgery. A novel, highly porous titanium material designed for bone in-growth and biological fixation1, Tritanium was first introduced to spinal surgeons in 2016 when Stryker’s Spine division launched the Tritanium...
This article is sponsored by EIT, LLC.
Emerging Implant Technologies (EIT): Cellular Titanium® for improved fusion results
Frustration at the clinical shortcomings of existing cage designs and materials on the market provided the impetus for the creation of EIT Cellular Titanium®....
Designed by Columbia Engineers, the robotic spine exoskeleton (RoSE) is the first device to take in vivo measurements of torso stiffness and to characterise the three dimensional stiffness of the human torso. RoSE could lead to new treatments for...
Neurokinex (charity number 1169964)—the first and only international community fitness and wellness affiliate of the Christopher and Dana Reeve Foundation’s NeuroRecovery Network (NRN)—has opened Neurokinex Kids to help children living with paralysis. This Gatwick-based facility provides children in the...
The International Society for the Advancement of Spine Surgery (ISASS) named Belgian surgeon Marek Szpalski (Iris South Hospitals/Molière Longchamp, Brussels, Belgium) society president for the year 2018—2019 at their annual conference last week (10–13 April, Toronto, Canada).
Szpalski is taking...
Two preclinical studies of IDCT, an allogenic (donor-derived), non-invasive cell therapy for the treatment of degenerative disc disease (DDD), demonstrate that proprietary discogenic cells, the active ingredient of IDCT, are safe and non-tumor forming. DiscGenics, a clinical stage regenerative...
Positive interim results of a retrospective analysis of the Luna 3D multi-expandable interbody fusion system were announced by Benvenue Medical at the Annual Meeting of the AANS/ CNS Section on Disorders of the Spine and Peripheral Nerves (14—17 March,...
The Tritanium C anterior cervical cage, a 3D-printed interbody fusion cage from Stryker’s Spine division intended for use in the cervical spine, has been implanted by 311 surgeons in more than 1,770 procedures across the USA since its introduction...
Gauthier Biomedical, a manufacturer of orthopaedic instruments and medical devices, has announced that it is the first company to achieve 510(k) approval by the US Food and Drug Administration (FDA) to market an electronic torque indicating device. Intellitorq (ITQ)...
Mainstay Medical, a medical device company focused on bringing to market ReActiv8, an implantable restorative neurostimulation system to treat disabling chronic low back pain, has provided an update on its application for the admission of ReActiv8 to the Australian...
Life Spine, a privately held medical device company based in Huntley, Illinois, has announced the first clinical use of SENTRY 2 lateral plating system, by Tien Le (NeuroSpine Center, Tampa, USA).
The SENTRY 2 lateral plating system, along with the...
RTI Surgical celebrates a significant global milestone: providing more than eight million biologic implants processed through the company’s proprietary sterilisation processes with zero confirmed incidence of implant-associated infection. In 2017 alone, RTI distributed more than 600,000 biologic implants—helping surgeons...
CTL Medical has recently secured clearance from the US Food and Drug Administration (FDA) to market its new Seurat Universal pedicle screw system for the practice of spinal fusion surgery.
This is the second FDA clearance for the company in...
MRI analysis of patients who received intradiscal injections of human dermal fibroblasts shows significant improvement in disc height after six months, the latest evidence from SpinalCyte demonstrates. The company say this suggests there is a quantifiable regenerative process stimulated...
CTL Medical, a Dallas-based medical device manufacturing and service company, has secured US Food and Drug Administration (FDA) clearance and approval to market its new MATISSE Titanium-PEEK ACIF cage system with the company’s proprietary TiCro surface technology in the...
The NuVasive porous polyether-ether-ketone (PEEK) material shows minimal surface damage upon impaction compared to titanium-coated devices, concludes a study recently published in The Spine Journal.
NuVasive has announced the results of the study, which compares the impaction durability of conventional...
The Scoliosis Research Society (SRS) has partnered with Globus Medical, K2M, Medtronic, NuVasive, Zimmer Biomet, and the International Spine Study Group Foundation (ISSGF) to provide continued funding and research infrastructure for the Adult Symptomatic Lumbar Scoliosis (ASLS) II study.
The...
Analytic software developed by orthopaedic trauma surgeons at NYU Langone Health accurately identifies which middle-aged and elderly patients face a greater mortality risk following surgery for an orthopaedic fracture, according to a new study.
PersonaCARE is a predictive, deep-learning software...
In an elderly Chinese population, intervertebral disc narrowing over a four-year period is associated with the presence of osteoporosis, and is greater in women than men. These results, initially published in the journal Spine, were the conclusions of a...
Cerapedics, a privately-held orthobiologics company, has announced the company received approval from the US Food and Drug Administration (FDA) to initiate an investigational device exemption (IDE) clinical trial evaluating the safety and efficacy of P-15L peptide enhanced bone graft...
Despite claims that helmets do not protect the cervical spine during a motorcycle crash and may even increase the risk of injury, researchers from the University of Wisconsin Hospitals and Clinics in Madison found that, during an accident, helmet...
A new study, published in the journal Stem Cell Reports, has revealed that the human brain's tiniest blood vessels can activate genes known to trigger spinal motor neurons, prompting the neurons to grow during early development. The findings could...
Camber Spine, a leading innovator in spinal and medical technologies, has announced the first surgeries using the company's proprietary SPIRA™-C Open Matrix Cervical Interbody device, a unique, interbody fusion implant consisting of spiral support arches and Surface by Design™ technology.
The...
Spineology has announced that enrollment is now complete in the company's Spineology Clinical Outcomes Trial (SCOUT) clinical trial.
The SCOUT IDE, conducted under an FDA-approved protocol, is a prospective, multicentre non-randomised performance goal investigation, designed to evaluate safety and effectiveness...
Dimitri Filippiadis and Alexis Kelekis (both Department of Radiology II, Attikon University Hospital, Athens, Greece) here detail the history and future of percutaneous vertebroplasty and kyphoplasty, and discuss the findings of the 2016 VAPOUR trial. This demonstrated that patients...
A team lead by Denis Evseenko (Department of Orthopaedic Surgery, University of Southern California, Los Angeles, USA) hopes to delay or reduce the need for joint replacement surgery in osteoarthritis patients with an injection.
Osteoarthritis is also known as degenerative joint...
Charles Fisher (University of British Columbia, Canada) was honoured in 2016 as one of the top 28 spine surgeons in North America, a highlight of a career spanning four decades. Head of the Combined Neurosurgical and Orthopaedic Spine Programme...
Daniel Spencer is a business manager at Charlton Morris, an executive search firm specialising in the medical space. Here, he argues that 3D printing is transforming the spinal implant marketplace, and will play an ever-increasing role in shaping the...
Scientists at Cincinnati Children’s Hospital Medical Centre report an experimental molecular therapy that restores insulation around peripheral nerves in mice, improves limb function, and results in less observable discomfort. These results were initially described in Nature Medicine in February...
Spinal muscular atrophy (SMA) patients treated with SPINRAZA (nusinersen) have experienced stabilised or improved motor function, contrary to the natural course of the degenerative disease, Biogen and Ionic Pharmaceuticals have announced. This is the end of study result from...
Varun Rimmalapudi and Jeff Buchalter (both Gulf Coast Pain Institute, Pensacola, USA) respond to an article printed in the October issue of Spinal News International: "Radiofrequency denervation fails to provide clinically important chronic low back pain improvement". The results were...
Experts explain their approach to treating patients who are living longer with cancer that has spread to the spine, as the options for metastatic spine tumours increase.
Every kind of cancer can spread to the spine, yet two physician-scientists who...
Eli Lilly and Company has announced that Taltz (ixekizumab) met the primary and all key secondary endpoints in COAST-V, a Phase three study evaluating the safety and efficacy of Taltz for the treatment of Ankylosing Spondylitis (AS), also known as radiographic...
The results of a two-year study demonstrate decompression with coflex interlaminar stabilisation extends the durability and sustainability of a decompression procedure. coflex is the first and only motion-preserving, minimally-invasive treatment approved for moderate to severe spinal stenosis post-decompression.
The European...
EOS imaging, a company specialising in 2D and 3D imaging and data solutions for orthopaedics, has announced the installation of an EOS system at the Beijing Jishuitan Hospital, a top-ranking speciality orthopaedic hospital in China.
This installation follows the first two...
Simplify Medical, a company focused on cervical spinal disc arthroplasty and maker of the Simplify cervical artificial disc, has announced a second tranche of its Series B financing of US$23.25 million, completing the oversubscribed round totalling US$44.25 million.
The lead...
Bioventus, a global leader in orthobiologic solutions, has entered into an agreement with LifeLink Tissue Bank, a division of LifeLink Foundation, to co-develop a next generation bone allograft solution for use in spine and trauma surgery. Terms of the...
Spinal muscular atrophy sufferers are a step closer to accessing vastly life-improving treatment following the formal National Institute for Health and Care Excellence (NICE) invitation to Biogen to submit SPINRAZA (nusinersen) for assessment via the Single Technology Appraisal route....
Spine technology company Astura Medical has today announced the completion of the initial surgeries and full commercial release for its Bridalveil Occipital-Cervico-Thoracic (OCT) system. The first cases were successfully completed at multiple US hospitals.
David Ou-Yang (The spine centre,...
The Food and Drug Administration (FDA) has today issued the final rule on “human subject protection; acceptance of data from clinical investigations for medical devices”.
The rule updates the FDA’s standards for accepting clinical data from clinical investigations conducted...
The nerve circuits that enable people to walk first appeared more than 400 million years ago in fish whose descendants still walk the seafloor on their fins. This is the finding of a study led by researchers from NYU...
Orthofix International, a global medical device company focused on musculoskeletal healing products, has announced the 510(k) clearance and US limited market launch of the FORZA XP Expandable Spacer System.
Designed to restore normal disc height in patients suffering from degenerative...
Mount Sinai researchers have found a possible link between a poor diet and back injuries, especially in women. The study suggests that following a specific type of diet that excludes fast foods and highly processed foods could decrease vertebral...
Researchers report the successful use of a novel therapeutic strategy to enhance forelimb recovery in mice with chronically injured spinal cords. The findings were presented by Hidenori Suzuki (Department of Orthopaedics, Yamaguchi University graduate school of medicine, Ube, Japan)...
The first cases using the Triojection system to treat spinal disc herniations in Germany were recently performed, Minimus Spine Inc. has announced. The cases were performed by Thomas Vogl, (Institute for Diagnostic and Interventional Radiology, J.W. Goethe University Hospital,...
New research using unbiased serum proteomics, a state-of-the-art technique in orthopaedics, aims to predict which cervical radiculopathy patients will fail conservative treatment and consequently require surgery. The principal investigator Steven Presciutti (Department of Orthopaedics, Emory University School of Medicine,...
Johnson & Johnson has formed the Johnson & Johnson Institute, bringing together 26 professional educational facilities across four continents.
The Johnson & Johnson Institute also includes “a network of online education and partnerships across multiple specialties,” says Ian Davies, vice...
Kleiner Device Labs has announced full commercial availability of a new spinal bone graft delivery tool, the KG 1, featuring a patented design that facilitates less-invasive procedures and has been proven in clinical testing to reduce spinal fusion failure...
Life Spine announced on 16 January that revenues for ProLift Expandable Spacer System grew by 493% for 2017 as compared to 2016.
“With the introduction of ProLift to the market in 2016, we have consistently experienced monumental sales growth...
Spineology has announced the completion of the first post-market study cases using its recently FDA-cleared Duo Lumbar Interbody Fusion System. In October, the company announced the initiation of this prospective, post-market lateral interbody fusion study designed to evaluate patient...
Founder and president of the International Spine Study Group Foundation, Shay Bess credits the friendships and research opportunities he has had through his career with the meaning and satisfaction he has found through his work. He speaks to Spinal...
New research has identified that inciting events may be associated with a positive outcome for patients diagnosed with lumbar facet joint pain. Charles Odonkor (Associate Faculty, International Rehabilitation Forum, Johns Hopkins University, Baltimore, USA) presented the findings at the...
Highlights:
New imaging technique to improve assessment of surgical patients with lumbar degeneration
Precipitating events associated with successful radiofrequency ablation of lumbar facet joint pain
Peptide enhanced bone graft outperforms autograft at two years
Profile: Shay Bess
https://spinalnewsinternational.com/wp-content/uploads/sites/11/2018/01/45-Spinal-News_lowres_US_v1.pdf
Highlights:
New imaging technique to improve assessment of surgical patients with lumbar degeneration
Precipitating events associated with successful radiofrequency ablation of lumbar facet joint pain
Peptide enhanced bone graft outperforms autograft at two years
Profile: Shay Bess
https://spinalnewsinternational.com/wp-content/uploads/sites/11/2018/01/45-Spinal-News_lowres_EU_v1.pdf
RTI Surgical announced on 4 January that it has signed an agreement to acquire Zyga Technology, with closing subject to filing with the state of Delaware, USA. Zyga Technology is a spine-focused medical device company that develops and produces...
NuVasive announced on 4 January the launch of the company’s Coalesce Thoracolumbar Interbody Fusion Device as well as FDA 510(k) clearance for expanded indications of its Cohere Cervical Interbody Fusion Device. The launch and updated claims follow the NuVasive September...
Participants are now invited to submit abstracts to the EUROSPINE scientific secretariat for consideration for inclusion in the 2018 annual meeting, to be held 19–21 September in Barcelona, Spain.
EUROSPINE is Europe’s largest spine society, with over 1,000 members and...
This article is sponsored by Cerapedics, Inc.
A FAST, RELIABLE AND PROVEN ALTERNATIVE TO AUTOGRAFT
i-FACTOR peptide-enhanced bone graft is challenging the gold standard in bone grafting and revolutionising expectations for spinal fusion.
Bone graft substitutes are used extensively in spinal surgery...
A prospective, randomised study presented at the 32nd EUROSPINE annual meeting held 11–13 October in Dublin, Ireland by Gabriel Tender (Louisiana State University, New Orleans, USA) has confirmed results that show minimally invasive techniques in transforaminal lumbar interbody fusion...
EOS imaging, a provider of imaging and data devices and software, has been chosen as this year’s winner of the Galien Foundation’s Prix Galien award in the medical device category. The Prix Galien recognises scientific innovation that improves patients’...
Camber Spine has announced the clearance by the US Food and Drug Administration (FDA) of its Spira-C Open Matrix cervical interbody device, a fusion implant that utilises the company’s Surface By Design surface enhancement technology. Spira-C is Camber Spine’s...
A surgical solution designed to “simplify discectomy in minimally invasive spinal fusion surgery,” the Concorde Clear from DePuy Synthes has this week been released across Europe, the Middle East, and Africa (EMEA).
“Concorde Clear is an ideal tool to enhance...
Omnia Medical has announced that the US Food and Drug Administration (FDA) has cleared its new vertebral body replacement (VBR) system, which is manufactured from PEEK-OPTIMA HA Enhanced polymer (Invibio Biomaterial Solutions). The system is designed for use in...
A report from Transparency Market Research estimates that the total value of the spinal fusion device industry will reach US$11.0 billion (£8.2 billion, €9.3 billion) within the next eight years. The increase will be primarily fuelled by the rising...
South Korean biomaterials company CG Bio has received a ‘World Class Product of Korea’ award from the Ministry of Trade, Industry and Energy and the Korea Trade and Investment Promotion Agency (KOTRA) in recognition of its “world-class” status. The...
The majority of patients studied were pain free after receiving a new image-guided pulsed radiofrequency treatment for low back pain and sciatica, according to research presented at the annual meeting of the Radiological Society of North America (RSNA; 27...
A prospective, multicentre trial has found that a peptide-enhanced bone graft is non-inferior compared to local autograft bone. In addition, the bone graft outperformed autograft bone for overall success measures.
The randomised single-blinded study reports that the use of I-factor...
This case report is sponsored by INVIBIO™
To view this case report as a PDF, please click here.
Timothy Bassett, MD (SouthEastern Spine Specialists, Tuscaloosa, AL, USA) has over 23 years of experience in spinal surgery, predominantly in the treatment of...
Analysis of a 3.3 million-year-old fossil skeleton has revealed the most complete spinal column of any early human relative, including vertebrae, neck and rib cage. The findings, published in the Proceedings of the National Academy of Sciences, indicate that...
The major gap between fusion-oriented spinal surgeons and pain management physicians is bridged by endoscopic spinal surgeons. Current surgical philosophy by traditionally-trained spinal surgeons focuses on fusion as the ultimate “cure” for a painful spinal segment caused by instability,...
The golfing world’s eyes are trained on the Hero World Challenge, which begins this week in the Bahamas. In April, Tiger Woods underwent his fourth back surgery in three years, and he now appears pain-free and ready to compete.
Woods has...
Park Ridge Health (Hendersonville, USA) has been awarded a Gold Seal of Approval for its certification as a Spine Center of Excellence from the Joint Commission, a recognised non-profit that accredits and certifies healthcare organisations and programs across the...
Medicrea yesterday announced the clearance of their additively-manufactured IB3D titanium interbody devices by the US Food and Drug Administration (FDA), and introduced a new technology, the Adaptek service for designing surgeon-adaptive interbody devices.
Denys Sournac—president and CEO of Medicrea—says,...
The T1 slope was first reported in 2010 by Knott and is considered one of the key players in cervical balance. Many studies have verified the strong relationship between the Health-Related Quality of Life or surgical outcomes and T1...
The former professional football player is a leader of Russo Partners’ Sports–Health Alliance, a service connecting sports, health and medicine. In addition to interviews at EUROSPINE2017 (Dublin, Ireland, 11–13 October) and the North American Spine Society (NASS) annual meeting...
A retrospective review of patients who underwent elective spine surgery in New Haven, USA has shown that the Rothman Index (RI) may be used to predict adverse events following discharge. The authors, from Yale Medical School (New Haven, USA)...
Winner of the 2017 Outstanding Paper Award for Medical/Interventional Science at the 2017 North American Spine Society (NASS) annual meeting (25–28 October, Orlando, USA), new research into astronauts returning from the International Space Station (ISS) is likely to have...
The rapidly changing healthcare environment poses a substantial amount of challenges. In the USA, this environment has shifted from surgeon preference and surgeon-driven expenditures to hospital mandates and, by extension, government-run insurance policies, as a vehicle for establishing a...
Two presentations at the Scoliosis Research Society annual meeting (SRS; 6–9 October, Philadelphia, USA) have shed light on the genetic factors at play in the risk and progression of adolescent idiopathic scoliosis (AIS). Familial studies suggest that AIS is...
In 2014, the company received clearance from the Australian Therapeutic Goods Administration (TGA) to market their range of interbody fusion devices. Two anterior lumbar interbody fusion (ALIF) procedures were undertaken at Princess Alexandra Hospital in Brisbane, Australia.
Richard Laherty, spine...
Long-term outcome data for a low-profile artificial disc replacement (Prestige LP, Medtronic) has indicated that the device, when used at two levels, is associated with a higher rate of overall success than is anterior cervical discectomy and fusion (ACDF)...
The popularity of sacroiliac joint fusion has grown rapidly over the past five years, with SI-Bone’s Ifuse device leading the market. Recently approved by the US Food and Drug Administration, the company has released a new version of their...
Researchers from Northwestern University, Evanston, USA, have developed a type of supramolecular glycopeptide nanostructure which appears to be able to amplify BMP-2 signalling significantly. Testing their nanostructures, the team were able to reduce the amount of the growth hormone...
A Conformité Européene (CE) Mark has been received by Medtronic for its neurostimulation platform Intellis for spinal cord stimulation (SCS) and peripheral nerve stimulation (PNS) in the treatment of non-opioid chronic pain, including low back pain, allowing the device...
Paralyzed Veterans of American (Paralyzed Veterans) has announced the launch of its 2018 Education and Training Foundation grant cycle. Applications for awards up to US$50,000 (£38,000, €43,200) for projects that aim to improve the lives of veterans and all...
Tissue Regenix Group yesterday announced the immediate appointment of Steve Couldwell as Chief Executive Officer (CEO).
“I am delighted to be appointed CEO and look forward to leading the Group through the integration of CellRight and expansion of commercial activities...
Stim onTrack, an app from Orthofix International designed to help patients comply with bone growth stimulation therapy, has been announced as the winner of the 2017 Spine Technology Award, presented by Orthopedics This Week.
“We are honoured to accept this...
Over the past 10 years, wearable technology has filtered into the mainstream, with market-leading Fitbit counting over 23 million active users. Wearables are used for tracking general measures of health such as heart rate, physical activity and sleep. Neurosurgeon...
Running a busy spine surgery in the 21st century is not easy, writes Matthew Coffy. Healthcare professionals in all practice areas are facing unprecedented financial and operational pressures, and the challenge is exacerbated by the fact that physicians are...
The Levó head positioning system, developed by Mizuho OSI, was showcased at last week’s North American Spine Society (NASS) annual meeting in Orlando, Florida, USA. The positioning system—for procedures from cervical to sacrum—attaches to the company’s modular spine surgery...
“The United States is by far the largest consumer of these drugs, using more opioid pills per person than any other country … in the world,” said US President Donald Trump. He has declared the increase in opioid addiction over...
The North American Spine Society (NASS) annual meeting yesterday played host to the launch of two solutions by DePuy Synthes for simplifying minimally invasive surgery (MIS) procedures.
Minimally invasive surgeries now account for more than one-sixth of all spine surgeries...
Interim results from a world-first prospective study demonstrate that robotic-guided spine surgery results in a five-fold decrease in the rate of surgical complications, and in a seven-fold decrease in revision surgeries, compared to traditional minimally invasive surgery in the lumbar...
Medical device company Nuvasive have launched a new porous titanium interbody implant for their XLIF procedures, named the Modulus. The Modulus XLIF device is a fully porous device, developed using additive manufacturing technology, otherwise known as 3D-printing.
The new implants...
A study led by the University of Hong Kong (HKU), Hong Kong, is first to report that a new biomarker—the ultra-short time-to-echo disc sign (UDS), imaged using ultra-short time-to-echo magnetic resonance imaging (UTE MRI)—is significantly related to degenerative spine...
A US-first surgery to replace the sternum and part of the ribcage of a 20-year-old woman has been successfully completed by a team of surgeons at New York–Presbyterian Weill Cornell Medical Center, led by professor of clinical cardiothoracic surgery...
As his term as NASS president comes to a close, Spinal News International caught up with Todd Wetzel to discuss his career to date, his society achievements and his hopes for the future of the spinal field. Like many...
Life Spine have announced that their Sentry plating system, which has been designed to improve lumbar stabilisation in anterior lumbar interbody fusion (ALIF) procedures, has been released in alpha.
The Sentry ALIF incorporates a cam-style locking mechanism that prevents unscrewing,...
Life Spine have announced that their Sentry plating system, which has been designed to improve lumbar stabilisation in anterior lumbar interbody fusion (ALIF) procedures, has been released in alpha.
The Sentry ALIF incorporates a cam-style locking mechanism that prevents unscrewing,...
The Camber Spine presenters for next week’s North American Spine Society (NASS) annual meeting were announced by the company yesterday. Dr John Malloy IV (East Coast Orthopaedics, Pompano Beach, Florida, USA) and Dr Luis E Duarte (Texas Brain and...
Garen Wintemute (Violence Prevention Program, University of California Davis, USA), in an editorial in the Annals of Internal Medicine, has called for physicians to make a public commitment to talk to their patients about firearms, counsel them on safe...
A study from Weill Cornell Medical College (New York City, USA) has found that, in patients with lumbar spinal stenosis associated with degenerative lumbar spondylolisthesis, minimally invasive decompression may offer a better approach than open laminectomy.
The research team, led by Karsten Schöller,...
A team of researchers is planning a multicentre databank to track and evaluate the performance of personalised rods (UNiD Rod, Medicrea) in the treatment of scoliosis.
Manufactured according to individual patient imaging results using sophisticated planning software, anecdotal evidence suggests...
Eric Major, president and CEO of K2M, has been elected chairman of the company’s Board of Directors. The appointment is effective immediately.
Major has taken over from Dan Pelak, who served in the role from 2010. Pelak will assume the...
The US Food and Drug Administration (FDA) has cleared HD Lifesciences’ NanoHive interbodies. The company released the devices to the US market last month.
Each interbody device delivers “four clinical improvements,” compared to other comparable devices, according to Lucas Diehl,...
Highlights:
Sugar nanostructures reduce BMP-2 requirements for fusion by 99%
Radiofrequency denervation fails to provide clinically important chronic low back pain improvement
Profile: Todd Wetzel
Feature: Stem cells
https://spinalnewsinternational.com/wp-content/uploads/sites/11/2017/10/44-Spinal-News-International.pdf
Spineology has initiated a full market release of the Elite expandable interbody fusion system. More than 300 cases have been completed using Elite to date.
The Elite expandable interbody fusion system implant is inserted into the lumbar disc space at...
The US Food and Drug Administration (FDA) has granted 510(k) marketing clearance to Nexxt Spine for its Nexxt Matrixx system.
The devices leverage "Nexxt generation" technology to create interbody and vertebral body replacement devices with optimised open architectural porosity, residue-free...
Zimmer Biomet has officially launched the Avenue T transforaminal lumbar interbody fusion (TLIF) cage in the USA.
Avenue T incorporates VerteBridge plating. This is designed to facilitate simplified cage insertion and zero-profile, intradiscal fixation. It is intended to be...
DiscGenics has been notified by the US Food and Drug Administration (FDA) that an Investigational New Drug (IND) application for a clinical study of its first product candidate, IDCT, may proceed.
IDCT is a homologous, allogeneic, injectable cell therapy that...
The Blue Cross Blue Shield of Michigan has issued a coverage policy in favour of interlaminar stabilisation devices.
Titled "Interspinous/Interlaminar Stabilization/Distraction Devices (Spacers)," the policy is dated September 1, 2017. Paradigm Spine's Coflex is currently the only product that has...
A new White Paper from Medicrea has revealed very significant rod fracture reduction for the UNiD technology in comparison to traditional rods.
The company recently reached the 1,000-procedure milestone for its personalised rods.
Relative to manually-bent rods, patient-specific rods significantly reduced...
Timothy Bassett, MD (SouthEastern Spine Specialists, Tuscaloosa, AL, USA) has over 23 years of experience in spinal surgery, predominantly in the treatment of adult degenerative lumbar spine. Specialising in addressing failed lumbar fusions, Dr. Bassett has extensive experience in...
Expanding Orthopedics Incorporated (EOI) has received US Food and Drug Administration (FDA) 510(k) clearance for the FLXfit15. The device offers infinitely adjustable expansion, and lordosis correction of up to 4mm and 15 degrees.
The FLXfit15 is intended to expand the...
Waves of vertebrae-building signals pulse outward in mouse cells mimicking a developing embryo. Video: Pourquié lab
Researchers have been able to halt and restart the “ticking” of the vertebral segmentation clock in mouse cells. They speculate that this discovery could...
A study by Jun S Kim (New York City, USA) and colleagues has demonstrated that an artificially intelligent (AI) system can better predict risk factors for anterior lumbar surgery than logistic regression.
Statistical modelling is commonly used in clinical research to isolate...
The US Food and Drug Administration (FDA) has cleared Providence Medical Technology to market Ally in the USA.
Ally is a posterior fixation system, intended to provide immobilisation and stabilisation of spinal segments as an adjunct to fusion.
The system is...
Patient-specific titanium instrumentation has been implanted from the front and back, creating the first ever 360-degree personalised spinal surgery.
Orthopaedic surgeon Benjamin Taylor performed the two-stage procedure using Medicrea’s UNiD ASI system at Wellington Hospital, London. ASI stands for Adaptive...
The US Patent and Trademark Office (USPTO) has granted Implanet two new patents for the Jazz platform.
These patents bring the company’s total number of patents to 24 for the Jazz platform.
The platform includes the Jazz Lock, the Jazz Claw,...
Stryker’s Serrato pedicle screws have been implanted by more than 100 surgeons across the USA during the first 30 days of limited release.
A full commercial release is yet to take place.
The Serrato pedicle screw is the first dual-thread...
One-year results from the IMIA (Ifuse Implant System Minimally Invasive Arthrodesis) randomised controlled trial have shown better pain and disability improvements for SI-Bone’s Ifuse system than conservative care.
IMIA is a Level 1 clinical trial conducted at nine hospitals in...
The lumbar spine is the site of over 20% of injuries in Olympic sport, according to the British Association of Sport and Exercise Medicine. These high impact injuries can cause enormous trauma to the spine, in a group whose...
SpineGuard has promoted three employees with the goal of reaching operational profitability by the end of 2018.
Stéphane Bette, chief executive officer of SpineGuard, says, “We are very proud to announce these internal promotions as they reward highly talented members...
Highmark, the USA’s fourth-largest Blue Cross and Blue Shield-affiliated insurer, has expanded positive coverage of sacroiliac joint fusion devices. All devices cleared by the US Food and Drug Administration (FDA) for this indication, including cages or screws, with or...
New research led by The Ohio State University Wexner Medical Center, Columbus, USA, has found a potential non-antibiotic therapeutic strategy to prevent infections in patients with spinal cord injuries. This research using mice with spinal cord injuries breaks new...
Band-LOK has announced that two new patents have been granted by the United States Patent and Trademark Office (USPTO) regarding the company’s proprietary Tether Clamp and Implantation System.
Michael Albert, co-founder of Band-Lok, says, “We continue to explore additional clinically-relevant...
Brainlab has received US Food and Drug Administration (FDA) clearance for the Elements Spine stereotactic radiosurgery software programme. This software is designed to aid in the patient-tailored planning of radiosurgery treatments for indications of the spine.
Elements Cranial SRS has...
RTI Surgical has announced three new executive appointments. They include Jonathon Singer, a member of RTI’s board of directors, as chief financial and administrative officer, effective 2 October 2017.
Jonathon Singer will replace outgoing chief financial officer, Robert Jordheim. RTI...
NuVasive has received 510(k) clearance from the US Food and Drug Administration (FDA) for use of the company's redesigned Magec system with its Reline Small Stature system. The products were featured at the Scoliosis Research Society Annual Meeting (SRS;...
Implanet has received US Food and Drug Administration (FDA) clearance to market the new Jazz Passer. The technology comprises new passer instruments and a variation of the Jazz Band braid, the Jazz Passer Band.
According to a press release, the...
Mazor Robotics has announced CE mark approval for its Mazor X Surgical Assurance Platform. The approval allows Mazor and its commercial partner, Medtronic, to market the Mazor X in the European Union, and other countries that recognise the CE...
SpinalCyte, a tissue engineering technology company focused on regrowth of the spinal disc nucleus using human dermal fibroblasts, has announced today the issuance of Australian Patent No. 2015202319, “Methods And Compositions For Repair of Cartilage Using An In Vivo...
Titan Spine has appointed Chad Kolean as chief financial officer. In his role, Kolean will oversee Titan’s Finance Team and work to support the company’s growth following the launch of its NanoLock surface technology.
Prior to joining Titan, Chad Kolean...
Stryker’s Tritanium C anterior cervical cage, a 3D-printed interbody fusion cage intended for use in the cervical spine, has received 510(k) clearance from the US Food and Drug Administration.
The cervical cage is constructed from Stryker’s proprietary Tritanium in-growth technology....
Experts at Queen’s University Belfast, Belfast, UK, have designed a flexible battery that could provide an alternative to the rigid batteries that usually power medical implants.
Currently, devices such as pacemakers, defibrillators and neurostimulators are fitted with rigid and metal...
The ability of stem cells to differentiate into a plethora of distinct adult cells has led to great excitement in both scientific literature and lay media. After years of research, however, tangible evidence for their promise remains elusive. Are...
The US Food and Drug Administration has approved Medtronic’s Intellis spinal cord stimulator. The company has now launched the product in the USA for the management of certain types of chronic intractable pain.
The Intellis platform was designed to overcome...
Carevature has announced positive preliminary clinical results for its Dreal spinal decompression and bone removal system. The data were given in an oral presentation by John Peloza (Dallas, USA) at the annual forum of the Society for Minimally Invasive...
K2M has announced the global launch of the Everest Minimally Invasive (MI) XTower instrumentation—an enhancement to the Everest MI XT spinal system. The announcement took place at the Society for Minimally Invasive Spine Surgery Annual Forum (SMISS; 14–16 September,...
Spinal Resources has received US Food and Drug Administration (FDA) 510(k) clearance for its Swedge pedicle screw system. This system offers multiple features including titanium tulips, double lead and cortical cancellous threads; polyaxial, monoaxial, reduction, and iliac screws; and...
Jason Hannon has been announced as successor to Peter Crosby as chief executive officer of Mainstay Medical. The transition will come into effect on October 9, 2017. According to a company release, this move was planned in association with...
NuVasive has launched the LessRay software technology system commercially. Comprised of a propriety software algorithm and hardware components, it is designed to help address overexposure to radiation during operations.
Spine and orthopaedic surgeons can receive their lifetime occupational radiation limit...
Cerapedics has announced the publication of two-year follow-up data from a US Food and Drug Administration (FDA) Investigational Device Exemption (IDE) clinical trial of the I-Factor peptide-enhanced bone graft. The results, published in Neurosurgery, show the graft to be...
NuVasive has announced the company's acquisition of Vertera Spine, a privately-held medical device company developing and commercialising interbody implants for spinal fusion using patented porous polyetheretherketone (PEEK) technology. As a result of this acquisition, NuVasive is now the only...
Timothy Bassett, MD, of SouthEastern Spine Specialists and Brad Prybis, MD of Carrollton Orthopaedic Clinic share their early clinical experience with interbody fusion devices made from PEEK-OPTIMA HA Enhanced for cervical and lumbar spinal fusion. Michael Veldman, Global Strategic...
Medtronic has announced the launch of a long-term clinical study programme to collect prospective data on rhBMP-2 (Infuse) bone graft in posterolateral fusion (PLF) and transforaminal lumbar interbody fusion (TLIF) spine procedures.
The first patient has been enrolled in...
NuVasive has announced the expansion of its San Diego global headquarters, including the creation of an innovation centre of excellence. Surgeons from around the world will be educated and trained on the company’s latest spine technology and procedures.
NuVasive was...
Joimax has announced the grand opening of its new state-of-the-art Training and Education Center in Irvine, USA. The company will be holding its first workshop at the centre on 8–9 September 2017.
January 2017 saw the relase of a US Current...
The first cases have been performed with multiple methods of in-situ expansion using Life Spine’s product range.
“Surgical intervention for the degenerative spine is a multidimensional challenge,” says Thomas Scully of Tucson, USA. “At one level, my goal was to...
InVivo Therapeutics is to cease enrolment in its recently-announced cervical study of the company’s Neuro-spinal scaffold. The company has also halted its chronic spinal cord injury stem cell and gene therapy research programmes, and reduced its staff by 13...
SI-Bone has announced that Highmark, the USA's fourth largest Blue Cross and Blue Shield-affiliated insurer, has established an exclusive positive coverage policy for minimally invasive sacroiliac joint fusion using the Ifuse implant system.
Highmark, an independent licensee of the Blue...
The US Food and Drug Administration (FDA) has cleared Xtant Medical’s product line extensions for the Calix-C family of cervical interbody cages. The clearance provides for the addition of two larger footprints, for use with allograft.
The Calix-C is now...
Spine Wave is to launch the Proficient posterior cervical spine system. This marks the company’s entrance into the posterior cervical fixation market.
A full launch will begin in October, with the arrival of commercial-scale inventory quantities.
According to a press release,...
The Excelsius GPS robotic guidance and navigation system from Globus Medical has been 510(k) cleared by the US Food and Drug Administration (FDA).
The robotic system received CE mark in January of this year.
This platform technology is designed to support...
Expanding Orthopedics Inc (EOI), a medical device company focused on developing and commercialising expandable devices for spinal surgery, has been granted two additional patents by the United States Patent and Trademark Office (USTPO).
The patents cover elements of the...
An abstract offering an update on Minimus Spine’s ozone gas injection treatment for painful disc herniation has been recognised as a Cardiovascular and Interventional Radiological Society of Europe (CIRSE) 2017 “Featured Paper”.
Alexis Kelekis, an interventional radiologist and editor-in-chief of...
Renovis Surgical Technologies has announced it has received 510(k) clearance from the US Food and Drug Administration (FDA) to market posterior lumbar Tesera porous titanium interbody fusion systems.
These systems feature implants for direct posterior (PLIF) or transforaminal (TLIF) approaches...
For the past 30 years, the “conventional” T2-weighted magnetic resonance imaging (MRI) has been broadly used to diagnose low back pain. However, this is not a highly-sensitive or reliable tool and, as such, may not be useful to identify...
Since 2009, hospital intensive care units (ICUs) in the USA have witnessed a stark increase in opioid-related admissions and deaths. This is a finding of a new study led by researchers at Beth Israel Deaconess Medical Center's (BIDMC) Center...
The Asia Pacific spine surgery device market is booming, with MicroMonitor predicting it to grow to US$2,223.1 million by 2019—a compound annual growth rate of 10.5% from 2014. As companies develop breakthrough technologies and disrupt the status quo, capturing...
The biggest event in the spinal calendar, this year’s North American Spine Society (NASS) Annual Meeting, is fast approaching. The meeting (25–28 October; Orlando, Florida) will offer delegates the opportunity to witness breakthrough research, get to grips with cutting-edge...
Stryker’s Spine division has announced that its Serrato pedicle screw, intended for use in the non-cervical spine as part of the company’s Xia 3 spinal system, has received 510(k) clearance from the US Food and Drug Administration (FDA).
Serrato pedicle...
NuVasive has announced the appointment of Rajesh (Raj) J Asarpota as the company's new executive vice president and chief financial officer (CFO), effective September 1, 2017.
As a member of NuVasive's global executive team, Asarpota will be responsible for the...
Camber Spine Technologies has launched its Open Matrix ALIF device in the USA following the receipt of 510(k) clearance from the US Food and Drug Administration (FDA).
Spira is an interbody fusion implant consisting of spiral support arches and Surface...
Spinal News International caught up with pioneering veterinary surgeon Noel Fitzpatrick (Guildford, UK) at NSpine 2017. A developer of numerous spinal and orthopaedic procedures for animals, he explains the untapped benefits of collaboration between researchers and both human and...
Stimwave has received US Food and Drug Administration (FDA) 510(k) clearance for the first wireless, micro-technology neuromodulation device that can enable ongoing full-body MRI scans under certain scanning conditions for the relief of chronic peripheral nerve pain.
The StimQ Peripheral...
Bioventus has announced two changes to its executive leadership team. Greg Anglum has been promoted to senior vice president and chief financial officer. Anthony D’Adamio has joined the company as senior vice president and general counsel.
Anglum, who joined Bioventus...
Providence Medical Technology, a developer of cervical spine technology, has received regulatory approval from the Australian Therapeutic Goods Administration (TGA) for its DTRAX line of instruments and implants used in tissue-sparing posterior cervical fusion.
The TGA approval covers the GL-DTRAX...
K2M has acquired the exclusive license to a portfolio of 17 issued and pending patents for expandable interbody technology.
K2M has also announced its intent to integrate its 3D-printing technology, Lamellar 3D titanium technology, into new products developed with this...
Based on 12-month follow-up clinical data from its safety and feasibility study, Intralink-Spine has reported that the company’s Réjuve system has continued to effectively eliminate or reduce low back pain and disability associated with degenerative disc disease.
According to the...
Invivo Therapeutics has provided an update on the progress of the company’s INSPIRE study (Invivo Study of Probable Benefit of the Neuro-Spinal Scaffold for Safety and Neurologic Recovery in Subjects with Complete Thoracic AIS A Spinal Cord Injury), which...
ApiFix has received Australian Therapeutic Goods Administration certification through its distributor, Orthotech.
The ApiFix system will now be marketed in Australia for the treatment and correction of adolescent idiopathic scoliosis (AIS) using a minimally invasive surgical approach.
The ApiFix approach’s minimally...
Camber Spine Technologies has exceeded 150 implantations of the Enza zero-profile anterior lumbar interbody fusion (ALIF) device launched last July.
“We have been doing anterior spine reconstructions for over 20 years and have used a lot of different ALIF devices”...
AlloSource has announced the release of AlloFuse Select CM, a premium addition to the company's AlloFuse portfolio.
AlloFuse Select CM combines osteoconductive, osteoinductive, and osteogenic properties to initiate and nurture bone growth, delivering the benefits of autograft bone without the...
Globus Medical’s acquisition of KB Medical, a robotic developer based in Lausanne, Switzerland, has closed during the second quarter of 2017.
KB Medical’s AQrate robotic assistance system, indicated for precise positioning of surgical instruments and spinal implants during general spinal...
Life Spine has announced the first clinical uses of its TiBow minimally invasive transforaminal lumbar interbody fusion (TLIF) expandable spacer system featuring Osseo-Loc surface technology with James Lynch of Reno, USA, and John Anson of Las Vegas, USA.
TiBow joins...
Medicrea has announced that the world’s first minimally-invasive spine surgery using patient-specific implants has been performed by Christopher J Kleck at the University of Colorado Hospital (Boulder, USA) using the company’s UNiD Rod.
The limited visualisation of the spine associated...
The first surgeries using 4WEB Medical’s Lateral Spine Truss system have been performed across the USA. The lateral interbody fusion device is designed to solve known surgical problems associated with legacy annular implant designs, according to a company release.
"4WEB's...
Over half the required number of implants in Mainstay Medical’s ReActiv8-B clinical trial have been performed. The clinical trial is investigating the company’s novel multifidus stimulation device (ReActiv8), which is intended to help strengthen the muscle as a means...
Recently, scientists in the UK and Sweden developed a surgical technique to reconnect sensory neurons to the spinal cord after traumatic spinal injuries. They have now gained new insight into how the technique works at a cellular level by...
SurGenTec has been issued device and method patent No. 9,668,881 from the United States Patent and Trademark Office for its graft loading technology to post-fill implant cages.
The Graftgun is a universal graft delivery system that is designed to allow...
Vertera Spine has announced that the company’s Coalesce lumbar interbody fusion device has received US Food and Drug Administration (FDA) 510(k) clearance for use in anterior, transforaminal, posterior, and lateral lumbar interbody fusion procedures.
The device features Vertera Spine's proprietary...
CTL Medical has secured US Food and Drug (FDA) clearance to market its new Matisse titanium anterior cervical interbody fusion (ACIF) cage implant with the company’s TiCro surface, used in the practice of spine fusion surgery.
According to a company...
Following the terrorist attacks in Paris and Nice, French experts outline the country’s medical response to terrorism in The Lancet.
By drawing on expertise from the military, equipping emergency responders, and improving victim identification processes, the authors state that the...
Richard Grant has agreed to serve as an advisor to Spinal Simplicity, and will join the company’s Board of Managers.
Spinal Simplicity produce the Minuteman family of sterile-packed, posterior, non-pedicle supplemental fusion and fixation devices for use in the non-cervical...
ReNetX Bio has been launched as a new company aiming to develop the neuro-restorative Nogo Receptor platform technology developed at Yale University, New Haven, USA, by Stephen Strittmatter.
Strittmatter is scientific advisor to and co-founder of the new company.
The new...
SpinalCyte, a Texas-based tissue engineering technology company focused on regrowth of the spinal disc nucleus using human dermal fibroblasts, has been issued a new Japanese patent.
The technology described in the patent (No. 6151006, “Methods And Compositions For Repair Of...
Premia Spine has launched US Food and Drug Administration (FDA) pivotal study of an updated version of its Tops system. According to chief executive officer Ron Sacher, this is “the only posterior arthroplasty device for degenerative grade I spondylolisthesis...
Emerging Implant Technologies (EIT) has received full approval from the US Food and Drug Administration (FDA) to commercialise its spinal interbody product offerings for anterior, transforaminal and posterior lumbar interbody fusion procedures, and cervical procedures.
EIT cellular titanium is a...
ApiFix has agreed an exclusive distribution deal in Spain with Acuna-Fombona, an Iberian distribution company.
ApiFix has developed a minimally invasive, non-fusion spinal implant system for the correction of adolescent idiopathic scoliosis.
ApiFix is intended to improve the quality of...
Simplify Medical, maker of the Simplify cervical artificial disc, has closed Series B financing of US$21 million. The new funds will be used to complete two ongoing US pivotal clinical trials of the Simplify Disc, studying its use in...
Philip Breedon, professor of Smart Technologies at Nottingham Trent University (Nottingham, UK), takes Spinal News International through the latest developments in co-robotics and telerobotics, including his department's innovative Scolibot system. Scolibot uses one robot to track the movement of...
Spinal News International caught up with Max Aebi (Bern, Switzerland), executive chairman of eccElearning, at NSpine 2017. He discusses the ways that online learning platforms will transform medical education from large lecture-based conference teaching to digital lessons enriched by...
Dennis Cirino, NuVasive's vice president of Computer-Asissted surgery, spoke to Spinal News International at NSpine 2017 about the importance of alignment in both deformity and degenerative cases. He explains his role in developing a technology working to achieve alignment...
Spinal News International catches up with Bronek Boszczyk (Nottingham, UK), NSpine's director and course chairman, at this year's Main Conference. He talks about the importance of collaboration accross different clinical disciplines and industry, as well as the most promising...
At NSpine 2017, Vikas Kapoor (Stockport, UK) talked to Spinal News International about treatment options for facet joint pain. He discusses the potential benefits of ablating the facet capsule in addition to the medial nerve (Denervex, Medovex), as well...
Sonny Bal, president and chief executive officer of Amedica, spoke to Spinal News International at NSpine 2017 about the potential benefits of silicon nitride for spinal fusion implants. He discusses impressive fusion rates and mass, the material's antimicrobial nature...
Spinal News International caught up with Todd Wetzel (Philadelphia, USA), the current president of the North American Spine Society (NASS) at NSpine 2017. He discusses the society's educational opportunities, the importance of regulatory advocacy in an uncertain political climate,...
Dominique Rothenfluh (Oxford, UK) discusses the importance of considering alignment in spinal surgery at NSpine 2017. The alignment of the entire spine, he explains, must be taken into account to optimise outcomes. Rothenfluh takes us through strategies and devices...
Spinal News International caught up with with Behrooz Akbarnia (La Jolla, USA) at NSpine 2017 (12–15 July; London, UK) to talk about the challenges associated with treating early onset scoliosis. He talks about the specific problems faced by paediatric...
The results of a clinical study on the efficacy of Amniox’ Clarix 100 cryopreserved amniotic membrane in lumbar microdiscectomy surgery have demonstrated greater improvements in both pain reduction and function from six weeks post-surgery than a control group.
The research—which...
Pierre Schwich has been appointed chief financial officer of EOS Imaging, effective immediately.
Marie Meynadier, chief executive officer of EOS imaging, says, “We are pleased to welcome Pierre to our team. His extensive experience with healthcare and technology growth companies,...
David C Dvorak has stepped down as Zimmer Biomet’s president, chief executive officer and member of the Board of Directors after 10 years at the helm of the company. He will remain in an advisory capacity to the company...
Prosidyan, a developer of proprietary fibre-based bioactive glass products, has received US Food and Drug Administration (FDA) 510(k) clearance for its Fibergraft bone graft (BG) putty, a bone graft substitute for posterolateral spinal fusion.
Fibergraft BG pulty is the second...
K2M and the Mitsubishi subsidiary Medicalnext have signed a long-term, exclusive distribution agreement. MedicalNext will supply the Japanese market with K2M’s spinal products.
The terms of the agreement include a partnership of up to seven years.
In April, K2M secured registrations...
Data presented at the 35th Annual National Neurotrauma Symposium (Neurotrauma 2017; 9–12 July, Snowbird, USA) have demonstrated beneficial effects for SRK-015 antibody therapy (Scholar Rock) in a preclinical model of spinal cord injury.
The study results revealed that the company’s...
SeaSpine has announced the limited commercial launch of the Skipjack expandable interbody system. The first cases using the device, a press release reports, have been completed.
Skipjack is an expandable interbody system based on patented technology purchased as part of...
Michael Enxing has been appointed as vice president and chief commercial officer of Vertiflex.
Enxing will be responsible for leading the company’s sales, marketing, health economics/reimbursement and professional education strategies, related to the launch of the Superion indirect decompression...
Spinal Elements has appointed Jason Blain to the position of president and chief operating officer. In this new position, he will have responsibility for product development, quality, marketing, operations, and regulatory compliance.
Blain has 20 years of experience in orthopaedics....
Xenco Medical has announced the nationwide expansion of the ASC CerviKit, the compact delivery and storage platform for the company’s entirely disposable anterior cervical discectomy and fusion (ACDF) systems engineered from a durable composite polymer.
The ASC CerviKit includes all...
SeaSpine has announced the full commercial launch of the company’s Mariner posterior fixation system.
Mariner is a pedicle-based system featuring modular screw technology and accompanying instrumentation. Designed to reduce the number of trays needed for surgery, the system is intended...
Stryker has received US Food and Drug Administration 510(k) clearance for its MultiGen 2 radiofrequency (RF) generator. This product is designed to provide physicians with efficiency, control and reliability when performing radiofrequency ablation for the treatment of facet joint...
Novarad’s OpenSight augmented reality system has been used in what the company believes may be the first surgical use of Microsoft’s HoloLens. An automated percutaneous lumbar discectomy was performed by Wendell Gibby, neuroradiologist and chief executive officer of Novarad.
“This...
In a market booming with new technologies and therapies, spinal surgeons are spoilt for choice when it comes to implants and other devices. Stryker’s John Mayor talks to Spinal News International about emerging technologies, education and the evidence behind...
Expanding Orthopedics has been granted CE mark for the FLXfit 15 expandable cage. The FLXfit 15 can expand up to 4mm and is designed to enable controlled lordosis correction of up to 15⁰. There are two linear length options;...
James Cook University Hospital in Middlesbrough, UK, has been added as the country’s first clinical site for Invivo Therapeutics’ INSPIRE study (InVivo Study of Probable Benefit of the Neuro-Spinal Scaffold for Safety and Neurologic Recovery in Subjects with Complete...
CTL Medical has reached a national partnership agreement with G-21, an Italian-based biomaterials company. As a result, CTL Medical will now co-market G-21’s bone cement, kyphoplasty and vertebroplasty technologies in the USA.
Daniel Chon, president and chief executive officer of...
At the NSpine meeting (12—15 June, 2017, London, UK), Bronek Boszczyk (Nottingham, UK) discusses osteosynthesis of atlas fractures.
At the NSpine meeting (12—15 June, 2017, London, UK), Bronek Boszczyk (Nottingham, UK) discusses difficult odontoid fractures.
US President John Fitzgerald Kennedy (JFK)—the second-youngest man to hold the position in history—is not known for his ill-health. However, the president was faced by a number of health problems throughout is life, including scarlet fever, long-standing gastrointestinal disease,...
At the NSpine meeting (12—15 June, 2017, London, UK), Bronek Boszczyk (Nottingham, UK) discusses the technical steps involved in reconstructing lumbopelvic and sacral fractures.
SpineGuard has appointed Stéphane Bette, co-founder, chief technical officer and US general manager, as chief executive officer of the company effective 13 July.
Pierre Jérôme, who has served as chief executive officer since the company’s founding, will continue to serve...
At the NSpine meeting (12—15 June, 2017, London, UK), Bronek Boszczyk (Nottingham, UK) discusses multilevel tumour resection including dura.
Medacta International has introduced the MectaLIF Anterior Hybrid interbody fusion device, which was cleared by the US Food and Drug Administration (FDA) in February 2017.
The MectaLIF family of cages is designed to provide enhanced in situ stability, restoration of...
Nearly one in three competitive athletes experiences low back pain. According to a literature review in the Journal of the American Academy of Orthopaedic Surgeons, low back pain among elite athletes who play varsity or professional sports requires additional...
At the NSpine meeting (12—15 June, 2017, London, UK), Bronek Boszczyk (Nottingham, UK) discusses how sacral osteotomy and lumbopelvic rotation can improve pelvic incidence.
K2M has received 510(k) clearance from the US Food and Drug Administration (FDA) and CE marking for its Nile Proximal Fixation spinal system, a device specifically designed for proximal construct augmentation.
Nile Proximal Fixation is designed to address complex spinal...
A European transnational consortium led by Maastricht University (UM), Maastricht, The Netherlands, is to spend the next four years developing innovative bone implants, intended to become an alternative for repeat surgeries, prolonged medication use and donor tissue implementation following...
At the NSpine meeting (12—15 June, 2017, London, UK), Bronek Boszczyk (Nottingham, UK) discusses how he performs staged correction of adult kyphosis and spinal lengthening.
At the NSpine meeting (12—15 June, 2017, London, UK), Bronek Boszczyk (Nottingham, UK) discusses anterior spinal access in an extra lunch time session.
Life Spine has announced the full commercial launch of two additions to the Osseo-Loc spinal implant range.
The company’s ProLift expandable posterior/transforaminal lumbar interbody fusion spacer system and the Tibow expandable transforaminal lumbar interbody fusion spacer system join Life Spine’s...
In three randomised trials, treatment of chronic low back pain (CLBP) with radiofrequency denervation resulted in either no improvement or no clinically important improvement in pain, according to a study published by the Journal of the American Medical Association...
SpineGuard have announced an exclusive licensing agreement with Adin Dental Implant Systems for the use of the company’s Dynamic Surgical Guidance (DSG) technology in the field of dental implantology, expanding the use of the technology beyond spinal applications.
“Modern dental...
The UK’s Information Commissioner’s Office (ICO) has found that the Royal Free National Health Service (NHS) Foundation Trust (London, UK) did not adhere to the UK’s Data Protection Act when it provided patient details to Google DeepMind.
The Trust, a...
At the NSpine meeting (12—15 June, 2017, London, UK), Bronek Boszczyk (Nottingham, UK) discussed multi level and thoracolumbar ALIF via visceral rotation in adult deformity.
ChoiceSpine has launched a new biologics portfolio at its National Sales Meeting in Nashville, USA.
The Biologics product portfolio is marketed under two brands named after the Stratotanker plane that refuels military aircraft inflight.
Stratofuse is designed to fuel fusion in...
EUROSPINE has announced that, from 2017’s meeting (11–13 October; Dublin, Ireland), the organisation will no longer print paper copies of the final programme, or congress bags in an effort to reduce waste and support the environment.
According to the EUROSPINE...
DePuy Synthes has announced the US launch of the Viper and Expedium fenestrated screw systems. When used in conjunction with the company’s Confidence high-viscosity spinal cement, the screws are intended to restore the integrity of the spinal column in...
K2M’s Capri Small 3D Static corpectomy cage system has received 510(k) clearance from the US Food & Drug Administration (FDA). Capri Small 3D Static is a 3D-printed corpectomy cage, and the company's third product family to feature its Lamellar...
Meditech Spine has received 510(k) clearance for its Cure lumbar plating (LP) system from the US Food and Drug Administration (FDA). The system is designed to accompany the Meditech’s lumbar Talos fusion devices, which are manufactured with PEEK-Optima hydroxyapatite...
InVivo Therapeutics hasannounced that the UCHealth Memorial Hospital in Colorado Springs, USA has been added as a clinical site for the INSPIRE study (InVivo Study of Probable Benefit of the Neuro-Spinal Scaffold for Safety and Neurologic Recovery in Subjects...
DePuy Synthes has acquired Innovative Surgical Solutions, doing business as Sentio; a privately-held company based in Wixom, USA, that markets nerve localisation technology for spine surgery. Financial terms of the transaction have not been disclosed.
Sentio's platform is designed to...
The US Food Drug Administration (FDA) has cleared Spineology’s Rampart One anterior lumbar interbody fusion system.
Rampart One is designed to minimise the exposure and vascular retraction requirements associated with traditional anterior spinal fusion procedures. The system includes both standard...
InVivo Therapeutics has announced that two patients in the INSPIRE study of the Neuro-Spinal Scaffold have improved from sensory incomplete AIS B spinal cord injury (SCI) to motor incomplete AIS C SCI in their most recent INSPIRE assessments. These...
InVivo Therapeutics has announced that a new patient has been enrolled into the INSPIRE study (InVivo Study of Probable Benefit of the Neuro-Spinal Scaffold for Safety and Neurologic Recovery in Subjects with Complete Thoracic AIS A Spinal Cord Injury)...
Thomas Mosnier, chief scientific officer of Medicrea—manufacturer of personalised devices such as the UNiD Rod for scoliosis treatment—talks to Spinal News International about the opportunities 3D printing offers for the large-scale production of patient-specific spinal products.
What is the typical...
Slipped discs are the most common reason for attending a doctor’s appointment in Switzerland. Whilst surgery can address the pain associated with herniation, the disc remains degenerated. Veterinary surgeons investigating the potential to regenerate spinal discs using stem cells...
Creative Medical Technology has announced the filing of intellectual property covering data supporting the use of immune system cells for stimulation of perispinal angiogenesis as a means of treating patients with lower back pain, as well as supporting intradiscal...
Northwestern University (Evanston, USA) scientists have designed a sugar-coated bioactive nanomaterial—sulfated glycopeptide nanostructures—that could prove to offer a new gold standard for bone regeneration.
“Regenerative medicine can improve quality of life by offering less invasive and more successful approaches to...
Highlights:
- Porous PEEK demonstrates greater osseintegration than micro-textured titanium
- SMART trial shows nerve ablation “well-tolerated and effective”
- Janice Werbinski and Kim Templeton: The criticality of gender specific medicine in spine health
- Feature: Designing spines - innovations in 3D printing
- Profile: Keith DK Luk
https://spinalnewsinternational.com/wp-content/uploads/sites/11/2017/06/43-Spinal-News.pdf
The US Patent and Trademark Office has issued a new patent relating to Zyga SImmetry’s Decorticator, an instrument which is designed to allow surgeons to prepare the sacroiliac joint for fusion by creating bleeding bone and space for autologous...
K2M’s Mojave posterior lumbar (PL) 3D expandable interbody system has received 510(k) clearance from the US Food and Drug Administration (FDA). According to a company release, Mojave PL 3D is a first-to-market, 3D-printed expandable posterior-lumbar (PL) interbody system that...
Keith KD Luk converted his childhood love of handiwork into a medical degree and numerous fellowships, eventually specialising in spinal surgery. Over his career, he has researched, lectured and led societies around the world. His research in intervertebral disc...
Magnetic resonance imaging (MRI) results play a crucial role in patient diagnosis and treatment, as well as the decisions of insurance companies and other payers to approve procedures. A study conducted by Hospital for Special Surgery (HSS, New York...
Implanet has obtained marketing clearance from the US and European regulatory authorities to market its new Jazz Braid.
The new Jazz Braid is an updated version, designed in response to surgeon feedback since the initial product launch in late 2013....
SpineGuard has reached an exclusive distribution agreement with XinRong Medical Group for PediGuard in China, Hong Kong and Macau.
China’s spine market has become the world’s second-largest market after the USA and is expected to be worth over US$1 billion...
Chronic low back pain affects approximately 10% of US adults and has a greater impact on racial or ethnic minorities and in people of lower socioeconomic status. Physical therapy is the most common evidence-based, reimbursable, and non-pharmacologic therapy prescribed...
A new patient has been enrolled into InVivo Therapeutics' INSPIRE study (InVivo Study of Probable Benefit of the Neuro-Spinal Scaffold for Safety and Neurologic Recovery in Subjects with Complete Thoracic AIS A Spinal Cord Injury) at Allegheny General Hospital...
Medicrea has received 510(k) clearance from the US Food & Drug Administration (FDA) for UNID Hub, a data-driven digital portal for the company’s Adaptive Spine Intelligence (ASI) software.
The UNID Hub is designed to support the surgeon workflow, identify tendencies...
Public Health England (PHE) and the Royal Society for Public Health (RSPH) have published “Everyday Interactions”, a report which aims to support healthcare professionals to record and measure their public health impact.
The report and toolkit were developed in close...
K2M has launched the Sahara AL expandable stabilisation system, the company's first expandable product within its interbody portfolio. Sahara AL is the only lordotic expandable interbody device with integrated screw fixation on the market to help achieve spinal balance.
The...
As promising new therapies such as those directly targeting survivor motor neuron (SMN) are entering clinical trials for infants, children, and adults with SMA, researchers are searching for biomarkers in blood that can monitor their effectiveness. Investigators report in...
Meditech has announced the launch of its Talos lumbar interbody fusion device comprised of PEEK hydroxyapatite (PEEK-Optima HA Enhanced, Invibio Biomaterial Solutions)—the newest generation of the product—following US Food and Drug Administration (FDA) approval.
Meditech's Talos device is an intervertebral...
Spasticity is a common disorder experienced by patients with spinal cord injuries (SCI). Previous studies have shown that excitatory repetitive transcranial magnetic stimulation (rTMS) can reduce spasticity. In a new study published in Restorative Neurology and Neuroscience, researchers found...
The US Centers for Medicare & Medicaid Services (CMS) has issued a new ICD-10 code (10th revision of the International Statistical Classification of Diseases and Related Health Problems, US variant) for a radiolucent porous interbody fusion device. The new...
SI-Bone has launched its new Ifuse-3D implant in the USA following regulatory clearance by the US Food and Drug Administration. The new titanium device is engineered to mimic the shape of the triangular Ifuse implant, this time incorporating a...
Spinal News International is partnering with NSpine—the world’s most comprehensive spine review course—to offer live and on-demand meeting coverage from Monday 12 June. Spinal News International will be offering a FREE live stream of all talks taking place in Lecture...
The Eurasian Orthopedic Forum will take place from 29–30 June in Moscow, Russia. Two faculty members speak with Spinal News International about the state of spinal surgery in Russia.
As well as heading the departments of Neurosurgery and Neurosurgery Research...
Medovex has received CE mark approval for the Denervex system allowing the company to market the Denervex system in Europe.
The Denervex system is designed to denervate and removes capsular tissue from the facet joint in one single procedure. Treatment...
In a preclinical study published in Stem Cell Reports, researchers developed a stem cell-based therapy for generating skin grafts to cover myelomeningocele defects—severe congenital defects affecting the spine—before birth. They first generated artificial skin from human induced pluripotent stem...
Little scientific research has studied the causal effect of psychosocial factors on low back pain, largely because of the inability of scientists to control psychosocial stress as a variable. A cohort study from Fukushima Medical University School of Medicine,...
The dangers of radiation exposure to patients and operating room staff are well-publicised. With the advent of minimally invasive spine surgery, both patient and operating room staff may undergo more radiation exposure to satisfy increased intraoperative imaging demands. According...
Bioventus has announced that its full surgical orthobiologics portfolio is now available through Premier, a US health care improvement company comprised of 3,750 US hospitals and more than 130,000 other provider organisations throughout the country.
Bioventus Surgical offers a platform...
Three-dimensional printing—also known as additive manufacturing—is one of the most exciting platforms shaping technology across sectors as diverse as art, food production and medicine. In play since the early 1980s, it is only in recent years that the potential...
Expanding Orthopedics Incorporated (EOI) has received CE mark for the FLXfit 15, an enhancement to the existing FLXfit 3D expandable cage. The FLXfit 15 expands up to 4mm and enables controlled lordosis correction of up to 15 degrees. There...
Results from three studies presented at the 2017 French Spine Society Congress (SFCR; June 1–3, Lille, France) have demonstrated the short and long-term safety, efficacy and patient satisfaction with the Zimmer Biomet’s Mobi-C cervical disc.
Thierry Dufour (Paris, France) presented,...
Vexim has received regulatory approval from ANVISA (Agência Nacional de Vigilância Sanitária), Brazil’s National Health Surveillance Agency, in order to commercialise the company’s SpineJack in the country.
According to a company release, the approval will open a new opportunity for...
NuVasive has announced that Skip Kiil is joining the company as executive vice president, International. In this role, Kiil will oversee the company's international operations, reporting to NuVasive's chairman and chief executive officer, Gregory T Lucier, and serving on...
Aurora Spine has announced that it is to offer out-licensing opportunities for its Polyaxial Zip ISP (interspinous process) implant locking technology. The technology is covered by United States Patent No. 9,603,637.
"The combination of…‘One-Step’ locking mechanism, with no setscrew, and...
InVivo Therapeutics has announced that data from the Christopher & Dana Reeve Foundation NACTN Registry will be included in the Contemporary Thoracic SCI registry study (now called the “CONTEMPO Registry Study”), a complement to the ongoing INSPIRE study of...
Following terror attacks in Paris, France, Brussels, Belgium, London, UK, Berlin, Germany, and, most recently, Manchester, UK, experts at the European Federation of National Associations of Orthopaedics and Traumatology Congress (EFORT; 31 May–2 June, Vienna, Austria) have focused on...
Zimmer Biomet has begun the process of recalling its SpF Plus-Mini and SpF XL IIb implantable spinal fusion stimulators in the USA, “due to higher than allowed levels of potential harmful chemicals, which may be toxic to tissues and...
CE mark approval has been granted for the world’s first percutaneous injectable anchor system, SandShark. The Stimwave system is used to fixate the company’s wireless neurostimulator devices through a minimally-invasive outpatient procedure.
“A wireless system that enables clinicians to actually...
SurGenTec, a minimally invasive orthobiologics company, has announced that the United States Patent and Trademark Office (USPTO) has granted a new patent for its bone graft delivery technology.
The proprietary Graftgun delivery system is designed to enable surgeons to deliver...
K2M has announced the launch of the Mesa 2 Cricket, an extension to the company's Mesa 2 deformity spinal system. Cricket is designed to provide surgeons with the ability to efficiently complete challenging correction manoeuvres in all three anatomical...
Zimmer Biomet has issued a voluntary field action notice to users of some of its Rosa Spine, Rosa One and Rosa Brain models. Citing a software problem, the notice promises that a software update will be delivered on-site by...
Both surgical and conservative methods of treating chronic low back pain have reported variable scientific and clinical outcomes. New prospective, double-blinded, randomised, sham-controlled trial results presented at the annual meeting of the International Society for the Advancement of Spinal...
by Kimberly Templeton and Janice Werbinski
It has been 36 years since the publication of the “Physicians’ Health Study,” which showed that low dose aspirin decreased the risk of a first heart attack in the 50,000 male physicians studied.1 This...
Analysis of a 3.3 million-year-old fossil skeleton has revealed the most complete spinal column of any early human relative, including vertebrae, neck and rib cage. The findings, published in the Proceedings of the National Academy of Sciences, indicate that...
Unable to attend the NSpine Main Conference this year? Want to re-watch the latest educational content?
Spinal News International is partnering with NSpine to offer live and on-demand coverage of the course right here, from Monday 12 June. The world's most comprehensive...
Xtant Medical Holdings has entered into a licensing agreement with Sites Medical, for utilisation of their proprietary OsteoSync Ti technology, a best-in-class porous titanium scaffold.
OsteoSync Ti technology is a highly porous titanium scaffold. Its high friction coefficient is intended...
A study to help determine outcomes for open vs. minimally invasive posterior lumbar spinal surgery has been approved by the Western Institutional Review Board. The study is being run by LifeSpine and the Oklahoma Spine and Brain Institute (Tulsa,...
Spinal News International is partnering with NSpine—the world’s most comprehensive spine review course—to offer live and on-demand meeting coverage from Monday 12 June. Spinal News International will be offering a FREE live stream of all talks taking place in Lecture...
Additional findings at the six-month juncture from Intralink-Spine’s early safety and feasibility study of the Réjuve system have indicated that the device can effectively eliminates or reduces low back pain.
“We have demonstrated the safety of this device, and it...
Life Spine has announced the launch of its Prolift lordotic expandable interbody solution with the company’s Osseo-Loc technology.
Prolift lordotic allows for in-situ disc height restoration, for minimally invasive posterior lumbar interbody fusion, transforaminal lumbar interbody fusion and oblique approaches....
Following the success of last year’s NASSISMISS meeting, the two-day scientific meeting from the North American Spine Society (NASS), the International Society for Minimally Invasive Spine Surgery (ISMISS), and the Indonesian Spine Society (ISS) will return this autumn (15–16...
The Accuro automatic spinal navigation system (Rivanna Medical) significantly enhanced the accuracy of epidural and spinal anaesthesia placement compared to traditional landmark techniques, even for residents-in-training and patients with atypical spinal anatomy, according to three abstracts presented at the...
An EOS System (EOS Imaging) has been installed at The Scoliosis Center, a division of Advocare The Orthopedic Center, which is the third site within the New York University’s Langone Medical Center Healthcare System to offer the EOS System...
Tricare has established a written coverage policy for minimally invasive sacroiliac joint fusion surgery. Tricare is a regionally-managed US healthcare programme for active duty and retired members of the uniformed services, their families, and their survivors.
The policy provides coverage...
Inspired Spine recently presented on the oblique lateral lumbar interbody fusion (OLLIF) procedure at the International Society for the Advancement of Spine Surgery (12–14 April; Boca Raton, USA). Hamid Abbasi, who developed the OLLIF, discussed the procedure, including results...
Always seeking to engage with spine specialists in new and exciting ways, the North American Spine Society (NASS) has launched a new social media campaign through its Facebook page, combining light-hearted “Throwback Thursday” (#TBT) posts with inspirational quotes from...
KnowYourBack is the North American Spine Society (NASS)’s patient-focused education platform. Brainchild of Raj Rao (Washington, DC, USA), it has been providing valuable information to individuals concerned about back pain and other spinal problems since its launch at the...
Since 2014, the SpineConnect forum has offered North American Spine Society (NASS) members the ability to share, discuss and debate cases, techniques, and research online. An innovative resource, the website offers academic and clinical collaboration in any place, at...
Intending to become a cardiac surgeon, Lisa Ferrara began her career as a biology student in Bridgewater, USA. A lecture on future technologies and prosthetics captured her imagination, leading her towards the innovative and exciting world of micro-electrical-mechanical systems...
IZI Medical Products has acquired Cook Medical’s vertebroplasty family of devices. The portfolio currently includes Duro-Ject Osteo-Site, Osteo-Force and Vertefix brands, consisting of needles, injectors and cements.
These products are used in the growing market for treating vertebral compression fractures...
Putting a spotlight on the latest innovations in spinal technologies, this year’s North American Spine Society (NASS) Summer Spine Meeting will take place in San Diego, USA, from 27–29 July. The meeting—which is chaired by Clinton Devin (Nashville, USA)...
The US Food and Drug Administration (FDA) has granted 510(k) clearance to Life Spine for its Cranial Fusion system. This clearance expands the indications for utilising the company’s Solstice polyaxial screws into the cervical spine.
The Cranial Fusion system is...
Premia Spine has secured US Food and Drug Administration approval for its pivotal study of the new Tops system.
“We are excited about the opportunity to provide USA patients with access to the only posterior arthroplasty device for degenerative grade I spondylolisthesis...
World-renowned golfer Tiger Woods has announced on his website that he has undergone a minimally invasive anterior lumbar interbody fusion (MIS ALIF) procedure to treat “ongoing pain in his back and leg”. Richard Guyer of the Texas Back Institute,...
Titan Spine has announced that Ed Graubart has joined the leadership team as vice president of Professional Development. Graubart will be responsible for enhancing and building the company’s infrastructure for training and professional development at the company.
Ted Bird, chief...
Vertebral Technologies (VTI) has partnered with Turkish medical distribution company Medikon to distribute its InterFuse product line in the country.
“We are excited to start working with Medikon, the company has been serving the Turkish spine surgeon community since 1995...
A new patient has been enrolled into InVivo Therapeutics’ INSPIRE Study (InVivo Study of Probable Benefit of the Neuro-Spinal Scaffold for Safety and Neurologic Recovery in Subjects with Complete Thoracic AIS A Spinal Cord Injury) at Oregon Health &...
The first Irish sale and implantation of Mainstay Medical’s ReActiv8 has taken place in Dublin. The implantation was performed at St Joseph’s Hospital, part of the Beaumont Hospital Group, in Dublin, by Josh Keaveny and Alexander Moudrakovski, consultants in...
Medtronic has updated the indications for use and the warning statements for its Navlock trackers, following two patient deaths. The trackers—which are part of its StealthStation navigation system—had been used alongside third-party instrumentation in a number of cases resulting...
New research detailing the molecular mechanisms involved in the breakdown of the soft tissue discs of the spine may provide opportunities for advanced, minimally invasive treatments for low back pain. The study investigated the molecular pathways which lead to...
A drug developed during World War II as an antidote for a chemical warfare agent has been found to be effective at suppressing a neurotoxin that worsens the pain and severity of spinal cord injury, suggesting a new tool...
The Eurasian Orthopedic Forum has announced that about 3,000 participants are due to attend its 2017 event in Moscow, Russia, participants from Russia, Southeast and Central Asia, Middle East, Europe and Americas. This will make it the largest event...
Implanet has been granted CE mark for its new Jazz Standalone implant, used in the treatment of adult degenerative bone disorders.
The device is designed to offer fast and simple freestanding posterior fixation to replace traditional fixation systems. It can...
The 50th installation of an EOS Imaging system has taken place in France at the Imanord medical imaging centre in the Hôpital Privé Villeneuve d'Ascq, a member of the Ramsay Générale de Santé network.
This new installation is the fifth...
Amedica has been granted marketing clearance for its Valeo interbody fusion devices in Australia.
The Valeo product line is made entirely of Amedica's proprietary medical grade silicon nitride ceramic—a composition intended to promote fusion with its nanostructured surface, osteoconductivity, osteoinductivity,...
The Si-Bone Ifuse minimally invasive sacroiliac joint fusion implant system has been used in more than 25,000 procedures worldwide, according to a company release. This total is helped by the increase in awareness of the sacroiliac joint as a...
Innovasis has become the first company to recieve US Food and Drug Administration (FDA) clearance for a standalone anterior lumbar interbody fusion (ALIF) system made from Invibio's PEEK-Optima HA Enhanced polymer.
The polymer is exposed on all surfaces of the...
Nexxt Spine has announced the full market release of the company’s Inertia Corti-Fixx cortical-cancellous pedicle screw system.
Corti-Fixx screws are designed to achieve greater cortical bone purchase with a smaller midline incision when utilised in the medial to lateral...
Patients undergoing spinal fusion surgery who are treated with methadone during the procedure require significantly less intravenous and oral opioids to manage postoperative pain, reports a new study published in Anesthesiology.
“This is a new application for an old pain...
Orthopaedic spine surgeon Jeffrey Goldstein, of New York City, USA has been named president of the International Society for the Advancement of Spine Surgery at the organisation’s 2017 annual meeting (ISASS17) in Boca Raton, USA.
Goldstein has been a member...
Scientists at the Gladstone Institutes (San Francisco, USA) have created a special type of neuron from human stem cells that could potentially repair spinal cord injuries. These cells, called V2a interneurons, transmit signals in the spinal cord to help...
Z-Medical has appointed René Rothacker as chief commercial officer and Jim Talbert as vice president of sales USA.
The company received US Food and Drug Administration (FDA) 510(k) clearance to market the minimally invasive Z-Pedicle screw system in 2015, and...
Transforaminal lumbar interbody fusion (TLIF) has been the "workhorse" spinal procedure for the past decade. However, there remains the challenge of suboptimal lordotic interbody cage placement. A group of researchers have proposed that adjustment of spinopelvic parameters play an...
Medtronic has launched its new StealthStation technology at the American Academy of Neurological Surgeons (AANS) annual conference in Los Angeles, USA. The new S8 model is intended to bring an advanced solution to neurosurgeons with enhanced workflows, efficiencies, and...
Titan Spine has announced the company has recently exceeded 1,000 implantations of its Endoskeleton titanium interbody fusion devices featuring Nanoolock surface technology since its launch in the fourth quarter of 2016.
Nanolock is the company’s next-generation surface technology featuring micro...
Si-Bone has announced the publication of a six-year study comparing the company’s Ifuse implant to both conservative management and radiofrequency denervation. The long-term study was published in the journal Neurosurgery.
The study evaluated 137 patients seen in an outpatient neurosurgery...
The first two installations of EOS Imaging’s EOS system are to take place in China at Nanjing Drum Tower Hospital , Nanjing, and Ruijin Hospital, Ruijin.
Nanjing Drum Tower Hospital, affiliated with Nanjing University Medical School, provides service to approximately...
7D Surgical has entered into exclusive sales representative agreements with two US medical device distributors; Surgical One and DB Surgical.
The 7D Surgical system is a machine-vision image-guided surgery platform. It is designed to quickly, easily register spine surgery patients,...
Vexim has launched its new Masterflow Plus treatment, targeted at low-energy vertebral compression fractures (A1-type according to Magerl and AOSpine Classifications) in osteoporotic bone, in Germany.
“Masterflow Plus is designed to…offer hospitals and physicians a valid alternative to treat osteoporotic...
NuVasive has announced the launch the new Reline Trauma portfolio, which is designed to provide surgeons the flexibility to customise their approach intraoperatively, including traditional open, Maximum Access surgery or hybrid procedures, depending on pathology and patient needs.
The system...
The virtues of polyetheretherketone (PEEK) as a material for spinal implants are well documented. PEEK’s radiolucency along with its similar strength and stiffness to bone have led to the widespread use of the material in implant manufacture. However, PEEK is...
New research published in Scientific Reports has shown for the first time that human intervertebral discs may strengthen in response to certain forms of exercise. Whilst degeneration has been associated with certain activities in the past, exercise has never...
DePuy Synthes has acquired 3D printing technology from Tissue Regeneration Systems (TRD). The 3D printing methods developed by TRS are designed to create patient-specific, bioresorbable implants with a unique mineral coating intended to support bone healing in patients with...
The president of the North American Spine Society (NASS), F Todd Wetzel (Philadelphia, USA), has announced the opening of the society’s 30-day public comment period for its draft coverage recommendations on allograft and demineralised bone matrix for spinal fusion...
The US Food and Drug Administration (FDA) has given 510(k) market clearance to Life Spine’s Plateau-C Ti cervical spacer system.
The system utilises the company’s proprietary titanium surface technology, Osseo-Loc, a surface technology designed to help create an environment for...
Zyga has announced the enrollment of its 100th patient in the Evolusion (EVSI) Clinical Study. This prospective, 40-site, 250-patient trial will evaluate long-term fusion and pain reduction in patients receiving SImmetry sacroiliac joint fusion.
"There is clear evidence demonstrating the...
Medovex, a developer of medical technology products, has entered into a partnership with Technology Consult Berlin (TCB) for distribution of its Denervex system throughout Germany. TCB is expected to provide sales, marketing and distribution services.
TCB—an associated partner of Kalms...
Inspired Spine reached the milestone of completing 500 oblique lateral lumbar interbody fusion (OLLIF) procedures. This minimally invasive surgical technique is designed to improve the performance of lumbar interbody fusions by delivering superior outcomes including less patient recovery time...
Zyga Technology has released 24-month computed tomography (CT) fusion and clinical results for its SImmetry sacroiliac joint fusion system. This prospective, multicentre study evaluated long-term fusion and pain reduction in patients receiving the system.
The study evaluated 18 patients for...
Amendia has announced the acquisition of Spinal Elements.
Spinal Elements’ clinically proven Ti-Bond porous titanium coating and upcoming product launches of the Lucent XP expandable cage and the Clutch interspinous process device, will be added to Amendia’s platform, including the...
Simplify Medical has announced that the first patient has been treated in the company’s pivotal clinical trial studying use of the Simplify disc in two adjacent levels of the spine as a treatment for cervical degenerative disc disease.
Composed of...
Safe Orthopaedics’ principal products for thoracolumbar spinal fusion have been listed by Assistance Publique - Hôpitaux de Paris (AP-HP; Paris, France). This is the main hospital authority serving Paris, France, and its suburbs.
This listing with AP-HP means that Safe...
Mazor Robotics has received US Food and Drug Administration (FDA) clearance for its Mazor X Align software. Mazor X Align is designed to assist surgeons in planning spinal deformity correction and spinal alignment for procedures performed with the Mazor...
The United States Trademark and Patent Office (USTPO) has granted Aurora Spine a patent related to its polyaxial Zip interspinous device.
The patent (number: 9,603,637) is entitled "Polyaxial Interspinous Fusion Implant and Bone Growth Stimulation System." This patent application covers...
Former Zimmer Bioment Product Development director, Brian May, has joined Spine Wave as executive vice president, Research and Development.
May was most recently one of the two directors running Product Development for the Zimmer Biomet Knee Reconstruction business. He joined...
Alphatec Spine has launched its new Battalion lateral system with the Alphatec squadron lateral retractor, and successfully completed initial patient surgeries including degenerative, multilevel and L4/L5 spinal segment cases.
Terry Rich, Alphatec Spine’s chief executive officer, says, “Our proprietary Squadron...
Aesculap has announced a new warranty program on its established portfolio of surface-enhanced interbodies for spinal fusion. This warranty announcement coincides with the launch of the TSpace XP interbody system, treated with PlasmaporeXP surface enhancing technology.
The warranty offers participating...
The UK's National Institute for Health and Care Excellence (NICE) has published their Interventional Procedure Guidance document for minimally invasive sacroiliac joint fusion surgery for chronic sacroiliac pain. The guidance recommends that the procedure be available to properly diagnosed...
A first-in-human trial has been expanded to add four more qualifying participants with chronic cervical injuries involving C5-C7 vertebrae. Launched in 2014 with the initial phase I study, this first-in-human clinical trial is evaluating the safety of neural stem...
Mayo Clinic (Rochester, USA) researchers have used electrical stimulation on the spinal cord and intense physical therapy to help a man intentionally move his paralysed legs, stand and make step-like motions for the first time in three years.
The case,...
SpinalCyte has received European Patent No. 1989289, “Methods And Compositions For Repair Of Cartilage Using An In Vivo Bioreactor.”
The technology described in the patent involves an in vivo bioreactor scaffolding for cartilage engineering and ex vivo methods of subjecting...
The first EOS platform (EOS Imaging) to be sold in Israel has been bought by Tel Aviv Sourasky Medical Center in Tel Aviv. The system is expected to be installed in April 2017 at The Dana-Dwek Hospital, one of...
The disruption of lumbar multifidus muscle control is one factor involved in many cases of functional instability and chronic low back pain. Whilst targeted movements can improve control of the muscle, a large number of patients cannot perform these...
The use of large registry-based datasets in spinal research has exploded in recent years. Often criticised for potentially limited clinical relevance, little research has been done on the academic impact of such research.
A team from Harvard Medical School, Boston,...
Value for money is an increasingly important part of hospital payer decision-making. In a The Spine Journal Outstanding Paper-winning study, a team from Toronto Western Hospital (Toronto, Canada) sought to investigate the lifetime cost-effectiveness of the surgical treatment of...
Highlights:
-One step closer to halting the "relentless cascade" of disc degeneration
-Pelvic incidence variable in 80% of healthy adults
-Feature: Conflict medicine
-Interview: Sanjog Pangarkar on the opioid crisis
-Profile: Lisa Ferrara
https://spinalnewsinternational.com/wp-content/uploads/sites/11/2017/04/42-Spinal-News-low-res-US.pdf
Highlights:
-One step closer to halting the "relentless cascade" of disc degeneration
-Pelvic incidence variable in 80% of healthy adults
-Feature: Conflict medicine
-Interview: Sanjog Pangarkar on the opioid crisis
-Profile: Lisa Ferrara
https://spinalnewsinternational.com/wp-content/uploads/sites/11/2017/04/42-Spinal-News-low-res-EU.pdf
Substance misuse is “one of the most pressing public health crises of our time” according to US Surgeon General Vivek H Murthy, in his November 2016 report on alcohol, drugs and health, Facing Addiction in America. Describing the nature...
Providence Medical Technology has announced recent 510(k) clearances from the US Food and Drug Administration for the standalone use of the Cavux cervical cage-L system as well as approval for its Ally facet screws.
The Cavux cervical cage-L system is...
Organogenesis is expanding beyond wound care with the acquisition of has acquired NuTech Medical’ biologics section in a move intended to expand the company’s focus from wound care.
According to a press release, the newly-combined company will offer a portfolio...
Z-Medical has announced that the first surgery using its minimally invasive surgery (MIS) Z-Pedicle system has taken place at the Chesapeake Regional Medical Center, Chesapeake, USA.
Grant A Skidmore, a neurosurgeon from Tidewater, USA, performed this first implantation. “The system...
Music therapy has been found to decrease pain in patients recovering from spine surgery, compared to a control group of patients who received standard postoperative care alone. The study, published in the American Journal of Orthopedics, included a team...
InVivo Therapeutics has received approval from the Toronto Western Hospital’s Research Ethics Board to enrol patients as part of its cervical spinal cord injury study. Toronto Western Hospital is the first study site for the company’s cervical spinal cord...
The US Food and Drug Administration (FDA) has granted 510(k) clearance to Nuvasive for the CoRoent small interbody system indicated for intervertebral body fusion at multiple contiguous levels in the cervical spine. This marks the first US clearance for...
InVivo Therapeutics has announced that the patient enrolled in January in the INSPIRE study of the Neuro-spinal scaffold has improved from a complete AIS A to an incomplete AIS B spinal cord injury in the time between the one-month and...
Burst Biologics has received institutional review board approval to begin a multicentre prospective clinical study in spinal fusion patients. This study will be conducted using BioBurst Fluid, a cellular allograft derived from umbilical cord blood. Fifteen clinical sites will...
Health Canada has approved Invivo Therapeutics’ Investigational Testing Authorization application to commence a clinical study of the Neuro-spinal scaffold in patients with acute, complete (AIS A) cervical (C5-T1) spinal cord injuries.
InVivo is currently in late-stage conversation with several site...
The completion of the first minimally invasive surgery using Spineway’s Mont-Blanc minimally invasive surgery product line has taken place in the USA. The operation was performed by Ludwig Orozco, a neurosurgeon in Dallas.
This first implantation was performed on a...
Safe Orthopaedics is expanding into the German market, and has appointed Jochen Esser as head of Sales for the country.
According to a company release, Jochen has over 25 years’ sales development and sales force leadership experience in the spinal...
Gary Henley has agreed to serve as an advisor to Spinal Simplicity and will join the company’s Board of Managers.
Gary Henley is a medical device executive with over 34 years of experience in the orthopaedic industry. Most recently, Henley...
Bioventus is to commission an innovative series of real-world evidence, direct-to-patient studies to validate the ability of its Exogen ultrasound bone-healing system to mitigate the risk of a fracture progressing to nonunion in the presence of known risk factors.
Recent...
People who lose a partner to suicide are at increased risk for a number of mental and physical disorders, including herniated discs, than those in the general population, new Johns Hopkins Bloomberg School of Public Health, Baltimore, USA, research...
A team of more than 50 clinicians at Advocate Children’s Hospital, Chicago, USA, have successfully separated a 10-month old patient from a parasitic rachipagus twin joined at the back of her neck. A few days following surgery, the hospital...
According to a “Best Paper” presentation at the North American Spine Society annual meeting (NASS; 26–29 October 2016, Boston, USA), it may be possible to develop a minimally-invasive, percutaneously-delivered combination of “cell-based bioactive factors” which can “mediate the progression...
The Syrian Civil War—now entering its sixth year—has displaced the greatest number of civilians of any conflict since World War II. Over a million people have sought refuge from the war in neighbouring Lebanon; a country with a population of...
Bone morphogenetic proteins (BMP), commonly used off-label to enhance paediatric spinal fusion (spinal arthrodesis), did not improve revision rates for paediatric spinal fusion, according to a study presented at the annual meeting of the American Academy of Orthopaedic Surgeons...
Medtronic has announced that the US Food and Drug Administration (FDA) has granted 510(k) clearance of Kyphon Xpede bone cement for fixation of pathological fractures of the sacral vertebral body or ala using sacral vertebroplasty or sacroplasty. This expands...
The US Food and Drug Administration has cleared Spineology’s Elite expandable interbody fusion system for a new size and an expanded indication. Included in the clearance is the addition of a narrower, 10mm, version of the device and an...
The first implantations of Spine Innovation’s expandable transforaminal lumbar interbody fusion device have taken place.
The first surgery was performed by James Bruffey and Robert Eastlack at the Scripps Healthcare System in La Jolla, USA. Bruffey comments, “The implant provides...
A new patient has been enrolled into InVivo Therapeutics’ INSPIRE study (InVivo Study of Probable Benefit of the Neuro-Spinal Scaffold for Safety and Neurologic Recovery in Subjects with Complete Thoracic AIS A Spinal Cord Injury) at the Keck Hospital...
People with spinal deformity also requiring a total hip replacement are at greater risk for dislocation or follow-up revision surgery, according to a new study. The research suggests that these higher-risk patients may benefit from a more personalised approach...
Updated guidance has been published by the National Institute for Health and Care Excellence (NICE) in the UK for lateral interbody fusion in the lumbar spine. NICE state that the evidence on efficacy for lateral interbody fusion is adequate...
The first US cases using the “one-step” insertion of pedicle “smart screws” guided by SpineGuard’s Dynamic Surgical Guidance (DSG) technology have been successfully performed. The surgeries were performed successfully by eminent surgeons throughout the USA.
For SpineGuard and Zavation, who...
Stryker will feature its 3D-printed Tritanium posterior lumbar (PL) cage at the annual meeting of the American Academy of Orthopaedic Surgeons (AAOS; 14-18 March, San Diego, USA), introducing a variety of new cage sizes.
Stryker’s Tritanium PL initially offered four...
K2M is to debut the Balance ACS platform at the Annual Meeting of the American Association of Neurological Surgeons/Congress of Neurological Surgeons Section on Disorders of the Spine and Peripheral Nerves (AANS/CNS Spine Summit; 8-11 March, Las Vegas, USA).
The system...
EOS imaging has sold a third EOS system to the Nemours Children’s Health System. The device was ordered by Nemours Children’s Hospital in Orlando, USA, one of the two flagship hospitals in the network.
The first two sales of the...
A new study published in BMC Musculoskeletal Disorders has found that the use of computed tomography (CT) imaging following spinal surgery has increased significantly. The study is the first to estimate the magnitude of CT imaging for the postoperative...
Ralph Mobbs, a neurosurgeon from the Prince of Wales Hospital, Sydney, Australia, has replaced two cancerous vertebrae with 3D-printed implants, according to a report from the Australian Broadcasting Company (ABC). The world-first surgery took approximately 15 hours, and involved...
LinkSpine has named Tom McLeer vice president of Sales and Marketing.
McLeer most recently served as senior vice president of US Commercial Operations for Alphatec Spine. Previously, he was chief medical officer and general manager of Spinal Operations for Pioneer...
Stryker has received US Food and Drug Administration (FDA) 510(k) clearance for its AVAflex balloon system. The device is available with Stryker’s bone cements and implants and the AutoPlex mixing and delivery system.
Chad Ludwig, marketing director at Stryker Instruments,...
Five times a day, roughly 1.6 billion Muslims worldwide, bow and kneel in the direction of the holy city of Mecca, Saudi Arabia, as part of the Islamic prayer ritual, the Salat.
According to research at Binghamton University, State University...
Safe Orthopaedics has announced that it is expanding into Germany, and has appointed Jochen Esser as head of Sales Germany.
According to a company release, Jochen has over 25 years’ sales development and sales force leadership experience in the spinal...
Alphatec Spine has launched its new Arsenal deformity adolescent idiopathic scoliosis (AIS) system and has successfully completed initial patient cases.
The Arsenal AIS system is intended to give surgeons a complete solution to address complex deformity pathologies, including uniplanar screws,...
Amend Surgical has received US Food and Drug Administration (FDA) 510(K) clearance to market NanoFuse BA as a bone graft extender for spine and orthopaedic applications.
NanoFuse BA is a composite containing 45S5 bioactive glass and a patent-protected carrier that...
Patients diagnosed with lumbar degenerative spine disease are more likely to receive the right care when a team of experts representing multiple medical specialties collaborate in reviewing the patient’s needs and determining the best treatment option, research has shown.
This...
Published in the European Journal of Pain, a study of 4390 Danish twins aged over 70 years old has found that those suffering from back pain had a 13% increased risk of all-cause mortality. The study investigated whether spinal...
The French patent office has granted Implanet a patent protecting the Jazz Lock implant in France.
A major component of the company’s band products for spine surgery, Jazz Lock is an implant designed to treat degenerative spine disorders.
Jazz Lock is...
GS Medical USA has announced the confirmed and pending launch of several US Food and Drug Administration (FDA) 510(k)-approved products.
The company plans to launch the AnyPlus anterior cervical interbody fusion implant and the AnyPlus dual-lead pedicle screw system in...
Joimax has obtained full product registration for its endoscopic minimally invasive spinal surgery platform from the Thai Food & Drug Administration.
This adds to its approvals in South Korea, China, Singapore, Indonesia, Hong Kong and Vietnam. For Malaysia, Taiwan and...
Wenzel Spine has completed the acquisition of the Primalok SP interspinous fusion system and the Primalok FF facet fixation system from OsteoMed.
The Primalok SP & FF platforms include a polyaxial interspinous process device and percutaneous facet screw system designed...
In this video shared by the Minimally Invasive Spine Centers of Excellence, Choll Kim (San Diego, USA) takes the viewer through a live laser endoscopic spine surgery. ...
Philips' new augmented reality system has been used in clinical practice for the first time at the Karolinska University Hospital, Solna, Sweden.
“This new technology allows us to use augmented reality in combination with 3D imaging for intraoperative surgical planning and...
Pelvic parameters are a “very hot topic” in spine, according to Howard Place, (St Louis, USA), lead author of a “Best Paper”-winning study on pelvic incidence, presented at the Annual Meeting of the North American Spine Society (NASS, 26-29...
The first clinical cases using a new surgical navigation system have been successfully treated at Karolinska University Hospital, Solna, Sweden.
The technology uses high-resolution optical cameras mounted on the flat panel X-ray detector to image the surface of the patient....
TranS1 has announced the release of the Capital bone graft harvester, a new device designed to allow for fast, reliable, and reproducible harvesting of autograft material from the iliac crest.
“Cancellous bone from the iliac crest is the gold standard...
Jeffrey R Binder has been appointed to the Minimus Spine Board of Directors. Minimus Spine manufactures the Triojection system for herniated spinal discs.
Binder was the chief executive officer of Biomet from 2007 until it was acquired for approximately US$14...
Paralysis is just one of the many serious health problems faced by patients who suffer spinal cord injuries, according to a report according to a report published on spinal cord injury published in Current Neurology and Neuroscience Reports.
Spinal cord...
Emergency patients treated with naproxen and placebo had outcomes as good as or better than patients treated with naproxen and diazepam for acute lower back pain, according to the results of a double-blind, randomised clinical trial published in Annals...
Safe Orthopaedics has launched a transverse connector designed to rigidify the stabilisation of posterior spinal osteosynthesis, as well as cement injectable through the Cypress screw to enhance its anchoring strength in osteoporotic or metastatic bone.
The injection of cement into...
The 2,000th implantation Nanovis’ Forticore has taken place as part of the company’s alpha product launch. The FortiCore posterior lumbar interbody fusion device comprises a deeply porous titanium scaffold interdigitated with a PEEK core.
“My patient’s short term response to...
K2M has introduced Balance ACS, a platform designed to apply three-dimensional solutions for spine patients. Balance ACS is intended to focus on achieving balance of the spine by addressing each anatomical vertebral segment with a 360-degree approach of the...
An evidence-based clinical practice guideline from the American College of Physicians published in Annals of Internal Medicine has recommended that physicians and patients should treat acute or subacute low back pain with alternative therapies such as superficial heat, massage,...
Alphatec Spine has appointed David H Mowry as a member of its board of directors. Mowry will be replacing Siri S Marshall. Mowry is qualified as an independent director under the definition established by NASDAQ.
Mowry is president and chief...
Camille Farhat has been named chief executive officer of RTI Surgical, effective March 15, 2017. He will succeed interim chief executive officer, Robert P Jordheim, who will resume his role as chief financial officer.
Farhat, 47, previously served as president...
Spinal Simplicity has received its third 510(k) clearance from the US Food and Drug Administration (FDA) for the Minuteman G3-R spinal implant, part of the Minuteman family of supplemental fusion and fixation devices. The Minuteman G3-R is intended to...
Vertebral Technologies (VTI), a minimally-invasive spinal implant medical device company based in Minneapolis, USA, has signed a group purchasing organisation contract agreement with St Louis, USA-based Resource Optimiation & Innovation (ROi), a provider-owned cost-management and supply chain-solution organisation.
This agreement...
All two-year follow up data for Axiomed’s USA lumbar Investigative Device Exemption clinical study has been collected and analysed, according to a company release.
Results from the lumbar European post-market assessment study showing clinically significant improvements in pain and disability...
DePuy Synthes, in collaboration with LifeNet Health, has launched ViviGen Formable cellular bone matrix, a second-generation cellular allograft designed to assist in the formation of bone during spinal fusion surgery.
ViviGen Formable joins the first-generation, ViviGen cellular bone matrix, which...
A minimally invasive surgical approach to spinal surgery is relatively new approach to the treatment of adult scoliosis. Studies have shown positive results in terms of deformity correction and complication rates. Little research has been published, however, on the...
In this video from the Journal of Neurosurgery, researchers present the case of a 53-year-old woman with "symptoms of both radiculopathy and myelopathy caused by a large, calcified disc herniation at C4–5."
After four months of medical treatment and rehabilitation,...
In 2017, NASS is collaborating with societies around the world to produce diverse and high-quality educational meetings. As well as at conferences in Guangzhou, Shanghai and Surabaya, expert faculty will be discussing the most important topics in contemporary spinal surgery...
The World Orthopaedics Innovation Summit & Expo 2017 (WOISE2017), the premier international event for innovations in orthopaedics (spine, joint, foot and ankle) and the high-tech life science industry, will be held in Shanghai, China, 8-9 April 2017.
In 2015, “Precision...
Enthusiasm for minimally invasive surgery has exploded in recent years, promising comparative results to open surgery with less blood loss and tissue damage for patients. Rapid technological innovation has created a plethora of new techniques and devices to feed...
In a value-based healthcare landscape, even when providing greater levels of evidence, US surgeons increasingly face coverage denials from insurance companies for proven spinal procedures and treatments. While spine care providers continue to raise concerns regarding this issue, it...
The 10th Congress of the Chinese Association of Orthopaedic Surgeons (CAOS) in Guangzhou, China this May will offer two sessions in collaboration with the North American Spine Society (NASS). The workshops will provide hands-on training with the latest ultrasound...
Many patients live with low back pain that radiates to the buttock, groin, thigh, and/or knees. The challenge for patients, and often their doctors, is determining the origin of the pain; the hip, the spine, or both. A new...
SpineGuard has been granted a patent by the US Patent and Trademark Office (USPTO) for the application of its Dynamic Surgical Guidance (DSG) technology to bone quality measurement.
“Because of population aging, orthopaedists and neurosurgeons are treating an increasing number...
ChoiceSpine has launched Harrier, an anterior lumbar interbody fusion system. The device was cleared for marketing by the US Food and Drug Administration in November 2016.
According to a press release, this will be the first of four new...
Rob Gronkowski, the New England Patriots’ famed tight-end, suffered his third disc herniation in seven years last November, bringing his participation in the 2016 American football season to an early end. Gronkowski is expected to be out of play...
Vexim has announced the expansion of its SpineJack patent portfolio in Asia. The company has received two new patents for SpineJack; one Chinese and one Japanese.
The SpineJack has undergone almost 47 patents applications gathered in three patent families. The...
SeaSpine has begun a full commercial launch of its Vu a.Pod prime nanometalene system.
The system features a zero-profile, standalone anterior lumbar interbody device that is designed to be configured in a variety of footprint and lordosis combinations to accommodate...
SpineSource has entered into a long-term exclusive distribution agreement with Kisco International to market, sell and distribute Kisco’s L-Varlock expandable lumbar cage for vertebral interbody fusion in the USA.
The cage is designed to provide spinal surgeons a device which...
Sacroiliac joint fusion has progressed markedly over the past five years. Positive results—the majority involving SI-Bone’s Ifuse device—have been reported in a growing number of studies. Interim results presented at the Society for Minimally Invasive Spine Surgery Annual Meeting...
Some of today’s most popular presents are smartphones. Thousands of these useful electronic tools were bestowed upon a loved one during the past couple of holidays. Many of us use them every day– a permanent companion.
“Text neck” is a...
The first commercial implantation of Mainstay Medical’s ReActiv8 has taken place in Germany.
The implantation was performed by Francis Kilian, orthopaedic and neurosurgeon at the Catholic Hospital Koblenz-Montabaur in Koblenz, Germany.
Kilian comments, "As spine surgeons we are always looking to...
With positive clinical results at the six-month juncture from its early safety and feasibility study in Malaysia, Intralink-Spine, has confirmed that the Réjuve system is now poised to begin its multi-site pivotal study beginning with sites in Southeast Asia.
“Two...
Exactech has sold its spine assets to ChoiceSpine, as part of a “restructuring” and “divesture” move. Terms of the transaction were not released. The company has also released Harrier, an anterior lumbar interbody fusion (ALIF) system
ChoiceSpine will now have access...
7D Surgical has received both 510(k) clearance from the US Food and Drug Administration (FDA) and a medical device license from Health Canada, enabling the North American commercial launch of its Machine-vision image guided surgery (MIGS) system for spine...
DePuy Synthes has signed an exclusive agreement in the US between DePuy Synthes Sales and Pacira Pharmaceuticals to co-promote Exparel, a long-lasting, non-opioid, local analgesic administered at the orthopaedic surgical site.
The agreement allows DePuy Synthes to promote Exparel across...
VTI has been granted a new patent from the US Patent and Trademark Office. This patent relates to the company’s modular in vivo assembly technology and its motion preservation, InterCushion pipeline product. VTI now has over 25 patents and...
DePuy Synthes has received 510(k) clearance from the US Food and Drug Administration (FDA) for the Viper and Expedium fenestrated screw systems. When used in conjunction with high-viscosity spinal cement, the screws are intended to restore the integrity of...
The US Food and Drug Administration has granted Camber Spine Technologies 510(k) clearance for the Siconus sacroiliac joint fixation system.
Daniel Pontecorvo, founder and chief executive officer of Camber Spine, says, "When used with the Prolix sacroiliac joint fusion system,...
PixarBio stock has been suspended from trade by the Securities and Exchange Commission, in line with the Securities Exchange Act of 1934. The Commission announced the suspension on 23 January 2017, issuing a statement that “the market for the...
Whale Imaging has received US Food and Drug Administration FDA 510(k) clearance for its G-Arm B6 Duo. The B6 Duo is the next generation of the G-arm with major improvements including axial tilt and greater table access.
According to a...
SpinalCyte, an engineering technology company focused on the regrowth of the spinal disc nucleus using human dermal fibroblasts, has announced the enrolment of its first patient in a study for the development of CybroCell, the first dermal fibroblast cell...
A new therapy to treat spinal cord injuries in people who have lost all motor and sensory function below the injury site has shown additional motor function improvement at six-months and nine-months following treatment with 10 million AST-OPC1. The...
Robotic surgery seems to offer a number of impressive benefits for spinal surgery, from increased precision and accuracy to potentially lower radiation doses for operating room staff and patients. With Globus’ Excelsius and Mazor Robotics’ Mazor X recently unveiled...
Minimally invasive surgery is generally considered to reduce blood loss and preserve surrounding
tissue, among other benefits. There is a dearth in the literature, however, on the association
between minimally invasive techniques and surgical site infections.
Research published in the Asian...
Implanet has been granted both US Food and Drug Administration (FDA) 510(k) clearance and CE marking to market its new Jazz Frame implant.
The Jazz Frame is a system of connectors for use in the Jazz Band technological platform dedicated...
An Invivo Therapeutics INSPIRE study patient enrolled in December 2016 has improved from a complete ASIA A spinal cord injury to an incomplete ASIA B spinal cord injury in the time between discharge and the one-month evaluation.
This is the...
Bioventus has named John Nosenzo as chief commercial officer. Nosenzo has worked in sales and marketing in the pharmaceutical and medical diagnostics areas markets for over 25 years.
He will be responsible for leading all global sales and distributor management...
New research has found spinal cord stimulation therapy can reduce or stabilise the use of opioids in patients battling chronic pain. Researchers examined opioid usage data from more than 5,400 patients both prior to and after receiving a spinal...
A single-centre retrospective study of patients who had undergone sacroiliac joint fusion has compared revision rates between those who received fusion via titanium triangular implants (Si-Bone Ifuse) or fixation via cannulated screws (7.2mm stainless steel screws, Synthes).
The study was...
Osseon has received a new US patent for a Steerable and Curvable cavity creation system, patent number US 9,510,885.
This new device will add to Osseon's line of existing steerable devices of bone augmenting products for treating compression fractures of...
Zebra Medical Vision has announced the latest algorithm to be included in its Deep Learning Imaging Analytics platform. The algorithm, capable of detecting vertebral fractures, is the latest addition to a line of automated tools that have been announced...
Is it a come-back for vertebroplasty? Two randomised controlled trials published in 2009 suggested that there is no clear benefit for treatment by vertebroplasty in comparison to a sham procedure. During this period, the US Administration praised the research,...
A new patient has been enrolled into Invivo Therapeutics’ INSPIRE Study (InVivo Study of Probable Benefit of the Neuro-spinal scaffold for Safety and Neurologic Recovery in Subjects with Complete Thoracic AIS A Spinal Cord Injury).
Travis Dumont, an INSPIRE...
Data obtained from a retrospective study involving the Spinal Elements’ Magnum+ device used in anterior lumbar interbody fusion have demonstrated a 96% rate of solid arthrodesis at an average of 7.3±2.3 months post-implantation.
The Magnum+ devices in the study were...
Precision Spine has received 510(k) clearance from the US Food and Drug Administration (FDA) for its ShurFit ACIF 2C anterior cervical interbody system. The device is made from PEEK and coated with both titanium and hydroxyapatite.
According to a company...
In this video shared by the Journal of Neurosurgery, researchers describe the surgical nuances of two-level cervical arthroplasty in a case of 2-level degenerative disease.
(Deshpande Rajakumar, Ankit Sharma, Akshay Hari, Subhas Konar, and Murali Krishna, Department of Neurosurgery, Fortis...
No longer only an image conjured by science fiction, bionic hands can return functionality in cases of traumatic nerve and muscle loss. A new article published in the Journal of Neurosurgery offers a treatment algorithm for identifying patients with...
The University of New Mexico Hospital (UNMH) in Albuquerque, USA, has been added as a clinical site for the INSPIRE (Invivo Study of Probable Benefit of the Neuro-spinal scaffold for Safety and Neurologic Recovery in Subjects with Complete Thoracic...
Effective January 23, 2017, Robert Delp will assume the position of president, Americas, and join the executive leadership team of the Zimmer Biomet. He is replacing Stuart Kleopfer, who has decided to retire from his position as President, Americas.
Delp...
SpineGuard has received 510(k) clearance from the US Food and Drug Administration (FDA) for its new DSG (Dynamic Surgical Guidance) integration module to be used in combination with Zavation’s spinal fusion system to make its pedicle screws “smart.”
The DSG...
Amedica has announced the successful completion of the first surgery using its Taurus pedicle screw system.
The surgery was performed by Thomas Scioscia, in Richmond, USA. Scioscia remarked on the features of the system, "The surgery went well and the...
Vertebral Technologies has announced the successful outcome of the first lumbar fusion procedure in Mexico using the Interfuse laterally expandable device.
Since September 2016, the company has partnered with Mexico-based medical product company BioMedical Technologies to bring their products to...
Medicrea has filed a 510(k) submission to the US Food and Drug Administration (FDA) for approval of the company’s 3D-printed titanium interbody devices, with compatible Unid Lab personalised surgical planning and analytical services.
Medicrea uses digital surgical modelling, combined with...
Robert Califf is to step down as US Food and Drug Administration (FDA) commissioner upon the inauguration of Donald Trump as US President on 20 January 2017. Democrat Califf, who was appointed to the post in February 2016 by...
The preliminary programme for NSpine (12-15 June; London, UK) has been published online.
The programme features sessions from BioSpine, the European Journal of Spine and EANS, as well as sessions focusing on conservative approaches, deformity, endoscopy, paediatric spine, trauma, sexual...
New research from The George Institute for Global Health (Sydney, Australia) has revealed that the weather plays no part in the symptoms associated with either back pain or osteoarthritis.
It is a commonly-held belief that episodes of both back pain...
Safe Orthopaedics has announced the launch of a bone substitute for its Walnut cage for cervical surgeries in Europe.
This device, developed in conjunction with a specialist in synthetic bone substitutes, aims to promote bone growth and thus to make...
The global market for spinal fusion—which includes spinal plating systems, interbody devices, vertebral body replacement devices, and pedicle screw systems (excluding minimally invasive spine devices)—is set to rise from approximately US$7.1 billion in 2016 to just under US$9 billion...
The US Food and Drug Administration (FDA) has formally reclassified pedicle screws from class III to class II devices. The reclassification came into force on 30 December 2016.
Following a 2014 proposed order to reclassify the systems, the FDA began...
Fewer veterans received prescriptions for risky dosages of opioid painkillers after a USA-wide initiative took aim at reducing high doses and potentially dangerous drug combinations, a new study has found.
Over a two-year period, high-dose opioid prescribing declined by 16%,...
The Cochrane Library has released a new review to the Cochrane Database of Systematic Reviews (CDSR) showing that yoga may lead to a small reduction in pain in people with chronic non-specific lower back pain over the short term....
Mainstay Medical has applied for ReActiv8 to be admitted to the Australian Register of Therapeutic Goods (ARTG) which would allow for commercialisation in Australia.
Mainstay’s ARTG application includes the results of the ReActiv8-A Clinical Trial, which showed clinically important, statistically...
Philips has announced the development of an augmented-reality surgical navigation technology, designed to help surgeons perform image-guided open and minimally-invasive spine surgery.
The technology uses high-resolution optical cameras mounted on a flat panel X-ray detector to image the surface of...
InVivo Therapeutics has announced that Rhode Island Hospital in Providence, USA, has been added as a clinical site for the INSPIRE (InVivo Study of Probable Benefit of the Neuro-Spinal Scaffold for Safety and Neurologic Recovery in Subjects with Complete...
LifeNet Health has begun production of ViviGen cellular bone matrix in its Northwest headquarters in Renton, USA. ViviGen, a cellular allograft developed by LifeNet Health, incorporates viable bone cells that support the healing process.
“Part of our mission at LifeNet...
Live-imaging and transcriptome analysis of medaka fish transgenic lines has revealed that exposure to microgravity can lead to the immediate alteration of cells responsible for bone structure formation. These findings, published in Scientific Reports, are important for assessing the...
Orthofix has received both US Food and Drug Administration (FDA) and CE mark approval for its next-generation CervicalStim and SpinalStim bone growth stimulators.
These class III medical devices use a low-level pulsed electromagnetic field designed to activate and augment the...
The St Michael’s Hospital in Toronto has been added as a Canadian clinical site for Invivo Therapeutics’ INSPIRE study (InVivo Study of Probable Benefit of the Neuro-spinal scaffold for Safety and Neurologic Recovery in Subjects with Complete Thoracic AIS...
SpinalCyte has received US patent number 9,533,024, “Methods And Compositions For Repair Of Cartilage Using An In Vivo Bioreactor.”
The technology described in the patent involves an in vivo bioreactor for cartilage engineering. The in vivo bioreactor will be biodegradable...
AxioMed have agreed a 15-year exclusive global license with DSM to use its proprietary viscoelastic material in the company's disc replacement technology.
Kingsley R Chin, managing partner of KICVentures and a board-certified spine surgeon believes this material will be revolutionary...
Globus Medical a leading musculoskeletal implant manufacturer, has announced that the Excelsius GPS, a system providing robotic trajectory guidance and navigation, has received CE mark.
This platform technology is designed to support both minimally invasive and open orthopaedic and neurosurgical...
The European Patent Office (EPO) has granted Implanet a European patent for its Jazz implant’s universal tensioning system.
This latest European patent concerns the Jazz implant’s tensioning system, which is the principal element of its instrumentation. It follows the patent...
Researchers funded by the US National Institute of Biomedical Imaging and Bioengineering (NIBIB) have developed a way to automatically label images of individual vertebrae during spinal surgery, preventing mistakes and saving surgeons both time and stress in the operating...
DePuy Synthes Products has announced an asset purchase and development agreement with Interventional Spine. Financial terms of the transaction have not been disclosed.
With Interventional Spine’s technology, DePuy Synthes will add a family of expandable cages to the company’s core...
Precision Spine has received US Food and Drug Administration (FDA) 510(k) clearance of its AccuFit lateral plating system.
The AccuFit plate is designed to provide optimal stabilisation with a low profile, titanium plating system that features four points of fixation...
NuVasive has received approval for instruments used in the Extreme Lateral Interbody Fusion (XLIF) procedure by the Japanese Ministry of Health, Labour and Welfare (MHLW).
Recent guidance from the MHLW requires dilators and associated components used in lateral access spine...
Amedica has announced results from a recent study showing rapid bone growth into porous silicon nitride. Explants of the company's porous silicon nitride from a large-animal model demonstrated bone healing into the material just four weeks after implantation.
"We anticipate...
Invivo Therapeutics has rebuked a press release from PixarBio claiming that it had made a US$77,000,000 stock offer to acquire the company.
The colourfully-worded release, dated 3 January 2017, claimed that PixarBio had initiated a take-over bid, expected to close...
The US National Institutes of Health (NIH) have recognised two spine-related studies as research highlights for 2016. Research into the effects of meditation and cognitive behavioural therapy on low back pain has been recognised as a “Clinical Breakthrough”, while...
PixarBio—developers of NeuroRelease, a morphine replacement, non-opioid pain treatment—has established the JP Reynolds Research Center in Woburn, USA.
The lab will focus on research and decvelopment products including:
Neuroscaffolds and injectable neuroscaffolds for acute and chronic spinal cord injury (SCI)
...
Carevature Medical has announced a 400 patient milestone for its Dreal line of products in the USA. Six centres in the US states of Massachusetts, New Hampshire, Connecticut and Texas, are currently taking part in Dreal's limited market release.
Carevature's...
Safe Orthopaedics has appointed a Scientific Advisory Board (SAB), made up of four European back surgeons.
The main tasks of the SAB will be to:
- Identify and assess trends in back surgery, particularly minimally invasive, percutaneous, fast-track and ambulatory...
A new patient with a T8-9 fracture dislocation injury has been enrolled into InVivo Therapeutics’ INSPIRE Study (InVivo Study of Probable Benefit of the Neuro-Spinal Scaffold for Safety and Neurologic Recovery in Subjects with Complete Thoracic AIS A Spinal...
EUROSPINE's interview with Christian Liebsch, winner of the meeting's 2016 Full Paper Award. ...
The US Food and Drug Administration (FDA) has issued a final rule to ban powdered surgical gloves, patient examination gloves and the absorbable powder used to lubricate surgical gloves.
Citing associations with serious adverse events—including allergic reactions, lung and airway...
In May 2017, NASS will run two intensive hands-on courses in Guangzhou, China as part of the 10th Annual Congress of the Chinese Association of Orthopaedic Surgeons.
The first course at the meeting is the “Ultrasound and Fluoroscopic Guided Lumbar...
What is most exciting about being NASS president?
One of the greatest pleasures of my 28 years as a member of NASS has been to see the society grow from its humble beginnings to its current position of preeminence; the...
This years’ Annual Meeting took place from 26-29 October in Boston, USA, and featured an extensive programme of scientific presentations, world-class speakers, and a bustling technical exhibition. Commenting on the highlights of this year’s meeting, past president Chris Bono...
by Allen Chen, MD, MPH
Continuing its global educational efforts, NASS successfully produced the inaugural Ultrasound and Fluoroscopic Guided Lumbar Procedures Workshop at the Singapore General Hospital on October 29-30 2016. This was the first freestanding NASS workshop, which was held...
The Canyons Village in Park City, Utah, USA will once again host the annual Evidence & Technology Spine Summit, 22-25 February 2017. In its 13th year, this meeting provides participants with a diverse educational programme and ample opportunity for...
The UK’s National Institute for Health and Care Excellence (NICE) has announced its participation in the US Food and Drug Administration (FDA)’s Payer Communication Taskforce. This programme aims to accelerate US patient access to new technologies by gathering evidence...
Safe Orthopaedics has received CE mark for a new version of its patented Oak screw, used to treat thoracic vertebral fractures.
Previously, 7.5mm, 6.5mm and 5.5mm diameter versions of the screw were available for lumbar vertebral fractures. With its smaller...
The UK's National Institute for Health and Care Excellence (NICE) has updated its advice on the treatment of low back pain in those over 16, recommending exercise in all forms as the best way to begin management. The guidelines...
RTI Surgical has announced that Robert P Jordheim, executive vice president and chief financial officer of RTI, has been named interim chief executive officer.
He succeeds Brian K Hutchison, who informed the RTI board of directors last August of...
Twelve-month clinical and radiographic data evaluating long-term fusion and pain reduction in patients receiving Zyga SImmetry sacroiliac joint fusion have shown average back pain scores at almost half pre-surgery levels among the 18 patients studied. The data were published...
Alexis V Lukianov has been named to Orthofix’s Board of Directors. Lukianov’s appointment expands the Board to 10 directors, nine of whom are independent directors.
Lukianov has held senior executive positions with companies such as Medtronic, SofamorDanek and Smith and...
SpinalCyte has received Institutional Review Board approval to begin clinical trials with its dermal fibroblast cell product, CybroCell, in the treatment of degenerative disc disease. SpinalCyte is now approved to begin randomised, placebo-controlled, double-blind Phase I clinical trials.
The clinical...
The Orthopaedic Institute for Children (OIC), Los Angeles, USA, has installed the EOS imaging system (EOS). This makes it the first standalone ambulatory care provider with this technology in the region.
“Our entire organisation is committed to delivering the best...
US veterans experience higher prevalence of pain and more severe pain—including pain—than nonveterans, with young and middle-aged veterans suffering the most, according to a new analysis of the US National Health Interview Survey (NHIS) by the National Center for...
Zimmer Biomet has launched the PrimaGen advanced allograft. According to a press release, this autograft substitute offers the same bone-healing elements as autograft, but without the risks associated with donor site morbidity or harvest site complications.
PrimaGen advanced allograft offers...
Below are the answers to Spinal News International's cryptic crossword. Thanks for your participation!
Across
4 Spinal News
7 Lateral
12 Diagnosis
15 Spinal cord
16 Ilium
17 Minimally invasive
Down
1 Screw
2 Fusion
3 Facet joint tropism
5 Stenosis
6 Decompression
8 Degeneration
9 Navigation
10 Lordosis
11 Discectomy
13 Autograft
14 Sacrum
The International Society for the Advancement of Spine Surgery (ISASS) has issued a policy statement recommending the coverage of lumbar decompression with interlaminar stabilisation in carefully selected patients.
The document, "ISASS Recommendations/Coverage Criteria for Decompression with Interlaminar Stabilization – Coverage...
InVivo Therapeutics has announced the resignation of Steven McAllister from the position of chief financial officer effective December 31, 2016. According to a company release, he has indicated he will be transitioning to a new opportunity at a privately-held...
The Canyons Village in Park City, Utah, USA will once again host the annual Evidence & Technology Spine Summit, 22-25 February 2017. In its 13th year, this meeting provides participants with a diverse educational programme and ample opportunity for...
The North American Spine Society (NASS), the largest spinal society in the USA, and Spinal News International have announced that they are to collaborate, beginning December 2016, on a number of editorial projects over the coming year.
Spinal News International will...
Researchers have developed a urine test revealing the presence of a neurotoxin that likely worsens the severity and pain of spinal cord injuries. According to the authors, this could lead to a new treatment tool.
The neurotoxin—acrolein—is produced within the...
A large group of societies—including the North American Spine Society—have signed a letter urging for the US Capitol to take action to tackle the country’s growing opioid crisis.
The letter, addressed to the US Senate and the US House of...
Spinal News International recently met with DePuy Synthes Spine’s Dan Wildman and Bill Horton to discuss the company’s aims, strategies, and upcoming product releases. Aiming to improve patient outcomes across the field of spinal surgery, the company has a...
Surface technology for interbody fusion implants is an area of massive growth in the spinal market, with a large number of device companies and academic institutions hunting for the best ways to optimise bone/device integration. Some have focused on...
Dennis DeVito has worked with Mazor Robotics’ Rennaisance system for over a decade. In advance of the commercial launch of the new Mazor X, he gives Spinal News International an exclusive first look at the technology, and explains the...
Implanet has announced the publication of a new white paper, which presents clinical results from a group of adolescents suffering from hypokyphotic thoracic scoliosis treated with Jazz sublaminar implants.
According to a company release, this study—co-authored by the Departments of...
The latest and largest study to investigate the connections between back pain and psychological illness in low- and middle-income countries was published this week in the journal General Hospital Psychiatry.
The research team—headed up by Patricia Schofield and Brendon Stubbs...
Australia’s first viscoelastic total cervical disc replacement case using the AxioMed Freedom device has been successful performed by Richard Laherty of Queensland Neurosurgery, Brisbane.
Laherty completed the procedure on Monday, November 28th at the Princess Alexandria Hospital in Brisbane, Australia....
The US Food and Drug Administration has cleared Xtant Medical’s Xsert lumbar expandable interbody system.
The Xsert system is an all-titanium interbody device that can expand in-situ. It is available in various sizes and lordotic angulations, in order to fit...
Cerapedics has been awarded a group purchasing agreement with Premier, a healthcare improvement company.
The new agreement allows Premier members, at their discretion, to take advantage of special pricing and terms pre-negotiated by Premier for I-Factor peptide-enhanced bone graft. The...
EOS imaging has announced the official opening of the first EOS site in South Korea at the Konyang University Hospital, Nonsan. The University Hospital serves an adult and paediatric population of seven million outpatients and 25 million inpatients on...
Medicrea’s patient-specific UNiD rod technology has now been used in more than 1,000 procedures, according to a company release.
Evalina Burger, of University Colorado Hospital (Aurora, USA), states, “We now realise how important it is to provide a specific alignment...
In this advertorial, Spinal News International talks to Richard Assaker (Lille, France) and Larry Khoo (Los Angeles, USA) about their experiences with SpineGuard's PediGuard Threaded Device with DSG (Dynamic Surgical Guidance), and the ways in which this innovative technology...
Scott Blumenthal (Plano, USA) is best known as the first surgeon to perform an artificial disc replacement in the USA. His pioneering research into these procedures has helped to change the landscape of back pain treatments in North America,...
Junyoung Ahn (Department of Orthopedic Surgery, Rush University Medical Center, Chicago, USA) and others report in The Spine Journal that continued surgical experience in the context of minimally invasive lumbar decompression, with or without discectomy, is associated with reduced...
A study published in the Asian Spine Journal has found that, unlike that reported in Western literature, the majority of neglected thoracolumbar injuries persisting in the underdeveloped and developing world are due to “inadequate treatment at the initial contact”....
Zimmer Biomet’s Mobi-C cervical disc prosthesis has become the most widely covered device for one- and two-level cervical disc replacement by commercial health insurers in the USA.
Mobi-C, acquired as part of Zimmer Biomet's combination with LDR in July, is...
The first early successful outcomes using the Cohere cervical interbody fusion device have been reported by Vertera Spine.
Cohere is Vertera Spine's first device featuring the company's patented porous PEEK (polyetherether ketone) Scoria biomaterial technology. Scoria's porous architecture is designed...
Tyber Medical has launched their lateral access system, a second-generation lateral PEEK/titanium (TyPEEK) composite interbody and lateral plating system.
The Tyber Medical lateral access retractor utilises a three-blade design. The approach and disc preparatory instruments were designed for multiple levels,...
The United States Patent and Trademark Office (USPTO) is to issue TheraCell a patent for its demineralised bone fibre (DBF) technology.
TheraCell's DBF technology is incorporated into two new demineralised cortical fiber allografts, AlloFuse cortical fibres and AlloFuse fibre boats,...
Spinal Simplicity has received a Health Canada Medical Device License for its Minuteman G3 and HA G3 spinal implants.
The Minuteman is a posterior, non-pedicle supplemental fixation device, intended for use at a single level in the non-cervical spine (T1-S1)....
The first procedures using Spinal Elements’ minimally invasive Katana lateral system have been successfully completed.
The Katana lateral system is a muscle-splitting system that is designed to overcome some of the inherent challenges in minimally invasive lateral surgery. Currently-marketed lateral...
RTI Surgical has received Us Food and Drug Administration (FDA) 510(k) clearance for the Streamline OCT occipito-cervico-thoracic system.
This clearance expands the indication for polyaxial screw placement to include the cervical spine, and also includes clearance for a dual...
Mazor Robotics has recieved three additional orders for the Mazor X system from a first time customer.
According to a company release, the new client is a major US regional institution in the Northeast of the country.
Ori Hadomi, chief...
InVivo Therapeutics has announced that a new patient has been enrolled into the INSPIRE study (InVivo Study of Probable Benefit of the Neuro-Spinal Scaffold for Safety and Neurologic Recovery in Subjects with Complete Thoracic AIS A Spinal Cord Injury)...
Minimus Spine has enrolled the 30th subject into its 50-patient randomised study comparing Triojection; a system intended to facilitate the treatment of disc herniations with an intradiscal injection of ozone gas to discectomy.
Bruce Frankel, professor of Neurosurgery at the...
A German spine clinic has reported the successful application and ease of use of OrtoWay’s Ortowell for lateral minimally invasive fixation in a complicated surgical corpectomy, according to a company release.
The OrtoWell device was used for the first time...
DePuy Synthes Spine has launched the Synfix Evolution system, a new implant for stand-alone anterior lumbar interbody fusion (ALIF). The Synfix Evolution system delivers biomechanical stability to promote fusion and restore function, coupled with instrumentation designed to optimise surgical...
NuVasive has launched a number of new spinal innovations, including the company’s expanded Integrated Global Alignment (IGA) platform, which now supports all spinal procedures, including cervical alignment.
In addition, the company has introduced proprietary image enhancement software that allows the...
A combination of nonsteroidal anti-inflammatory drugs and TNF-inhibitors may help slow down spine damage in ankylosing spondylitis, according to new research findings presented at the American College of Rheumatology 2016 Annual Scientific Meeting in Washington, DC, USA.
Recent research on...
SeaSpine has received 510(k) clearance from the US Food and Drug Administration (FDA) for its Shoreline ACS anterior cervical standalone system, featuring TruProfile technology, and its Mariner posterior fixation system.
TruProfile offers a low profile plate designed to minimise cephalad-caudal...
Providence Medical Technology's Cavux
Providence Medical Technology has announced the commercial launch of its new Cavux cervical cage-L and Dtrax spinal system-L.
The Cavix Cervical Cage-L is an intervertebral cage made of solid titanium alloy with a large graft window and...
Joimax has received 510(k) clearance from the US Food and Drug Administration (FDA) to market its Vaporflex and Legato electrosurgical probes with radiowave technology for open and endoscopic spine surgery.
The Joimax electrosurgical instruments are comprised of a series of...
For patients with degenerative spinal disease, surgery is more effective in reducing pain that interferes with sexual activity, compared to nonsurgical treatment, reports a study in the November 15 issue of Spine.
"Sex life is a relevant consideration for the...
Medicrea has implemented a lifetime warranty on its patient-specific UNiD technology. The warranty covers all UNiD thoracolumbar rods, UNiD cervical rods and all associated Medicrea components implanted in the USA from November 1, 2016.
Denys Sournac, president and chief executive officer,...
Patients with ankylosing spondylitis or psoriatic arthritis who take statins may have as much as a 33% lower mortality risk, according to new research findings presented at the 2016 American College of Rheumatology Annual Scientific Meeting in Washington, DC.
Researchers...
UCB and Amgen have announced results from the Phase 3 BRIDGE study showing that in men with osteoporosis, the investigational agent romosozumab resulted in significant bone mineral density (BMD) gains at the lumbar spine—and the total hip and femoral...
Titan Spine has announced that Ted Bird has joined the executive management team as chief commercial officer. According to a company release, Bird will be responsible for the company’s global sales, marketing, strategic partnering, and national target accounts.
Peter Ullrich,...
InterMed Resources and Z-Medical have come to a distribution agreement with US-based HealthTrust. InterMed will distribute spinal implants manufactured by Z-Medical (Tuttlingen, Germany) to HealthTrust, a group purchasing and total cost management organisation that serves nearly 1,600 acute care...
Mighty Oak Medical has received a second FDA clearance for its patient-specific, 3D-printed Firefly pedicle screw navigation, extending compatibility to essentially all currently cleared pedicle screw systems. A press release reports that the new clearance also extends the indications...
Highlights: -Adult spinal deformity surgery outperforms non-surgical treatment for pain, fuction, cosmesis and mental health -Spinal metastasis cases almost triple over two decades in Ireland -Feature: Surface innovation -Profile: Scott Blumenthal
https://spinalnewsinternational.com/wp-content/uploads/sites/11/2016/11/41-Spinal-News-low-res.pdf
Highlights: -Adult spinal deformity surgery outperforms non-surgical treatment for pain, fuction, cosmesis and mental health -Spinal metastasis cases almost triple over two decades in Ireland -Feature: Surface innovation -Profile: Scott Blumenthal
https://spinalnewsinternational.com/wp-content/uploads/sites/11/2016/11/41-Spinal-News-low-res_USA.pdf
The effects of surgical scars can be far-reaching and life-changing for many women. Understanding and quantifying the impact of scars is an important aspect of patient management, however, scar size or placement is not routinely considered as a prominent...
In a study of the incidence of cancer in a cohort of adolescent idiopathic scoliosis (AIS) patients treated 25 years previously, investigators have found that patients treated with bracing or surgery had an overall cancer rate of 4.3%. This...
A number of studies presented at the Scoliosis Research Society Annual Meeting (SRS; 21–24 September, Prague, Czech Republic) and at Eurospine (5–7 October, Berlin, Germany) have used two-year follow-up data from the Scoli-Risk-1 study to reveal a multitude of...
A new model for measuring frailty in adult spinal deformity (ASD) patients has been assessed by the International Spine Study Group (ISSG) for its ability to predict both perioperative and postoperative complications. The team found an association between frailty...
The first prospective study analysing the impact of depressive symptoms in surgically-treated severe adult spinal deformity patients on health-related quality of life outcomes has found that, whilst depressed patients began with “significantly worse absolute health-related quality of life scores”,...
A pre-clinical study of Stryker’s new 3D-printed, highly-porous Tritanium PL interbody cage has found that using the device can result in “statistically superior range-of-motion, bone in-growth pofile and greater average construct stiffness” in comparison to both PEEK-only cages, and...
Zimmer Biomet has announced results of a seven-year outcomes study demonstrating statistical superiority of its Mobi-C cervical disc prosthesis vs two-level anterior cervical discectomy and fusion (ACDF) in overall success. In the study, overall success required improvement in Neck...
K2M has launched its award-winning Cascadia interbody systems, featuring the company’s Lamellar 3D titanium technology in the USA at the 2016 North American Spine Society (NASS) annual meeting.
K2M also presented clinical background on its 3D-printed technologies. The company highlighted...
Life Spine has introduced two new transforaminal lumbar interbody fusion (TLIF) products at the 2016 annual meeting of the North American Spine Society (NASS; Boston, USA)—the TLIF Retractor and the Plateau-LO.
According to a company release, the TLIF Retractor was...
Ascential (Stryker), an implant and delivery solution for lower acuity spinal procedures in the ambulatory surgery centre (ASC) and hospital settings, has been introduced at the 2016 North American Spine Society (NASS) annual meeting in Boston, USA.
According to a...
Globus Medical has launched the Quartex occipito-cervico-thoracic (OCT) stabilisation system. According to a company release, the product offers a variety of solutions to the challenges associated with posterior OCT fusion, while delivering reliability and ease of use.
Quartex screw heads...
Genesys Spine has released the TiLock cortical spinal system, launching the product at the North American Spine Society’s 2016 annual meeting (NASS).
TiLock cortical spinal system is intended to offer midline screw placement with a medial/lateral trajectory to provide a...
DeGen Medical has introduced the Latitude-C Porous Ti cervical interbody spacer, an extension of the company’s Latitude-C product line. According to a company release, the device is designed to consider the Uncinate process of the cervical vertebra.
The lateral angled...
DiscGenics has been granted 9 new patents, expanding its intellectual property portfolio within both the US and globally to 24 issued patents. The new patents include two in the US and seven in European markets.
The patent portfolio provides coverage...
The US Centers for Medicare & Medicaid Services (CMS) has issued its 2017 Final Hospital Outpatient Prospective Payment System (HOPPS) and Ambulatory Surgical Center (ASC) payment rule for minimally invasive sacroiliac joint fusion.
The final payment rule shows American Medical...
A prospective randomised clinical study of Amniox’s Clarix regenerative matrix used as an adjunct to lumbar discectomy has found statistically significant improvements on Oswestry Disability Index and Short Form-12 (physical component scale) scores at six months and two years.
The...
Signus has received 510(k) clearance from the US Food and Drug Administration (FDA) for the new Diplomat pedicle screw system.
The Diplomat has been developed in cooperation with international spine experts. As a posterior fixation system, it is intended to...
4Web Medical has announced that the first surgeries using the company's Curved Posterior Spine Truss system (PSTS) for transforaminal lumbar interbody fusion procedures have been performed.
Jeffrey Wise, Blue Ridge Orthopaedic and Spine Center, who used the Curved PSTS upon...
During its 31st Annual Meeting, the North American Spine Society (NASS) announced the winners of its prestigious 2016 Recognition Awards. NASS presents four annual awards which recognise unique and outstanding contributions to the field of spinal care and research....
A nationwide analysis of metastatic bone disease—believed by the authors to be the first of its kind in the world—has found that cases of spinal metastasis have increased by 182% from 1994 to 2012. The study used the National...
Clinically-significant positive outcomes for the surgical treatment of adult spinal deformity have proven elusive for researchers, with many studies limited by the non-randomisation of participants. A new study from the European Spine Study Group, however, has found better clinical...
According to a new study presented at the 31st Annual Meeting of the North American Spine Society (NASS), tobacco use may harm the body’s healing response from surgery for cervical myelopathy based on number of cigarettes smoked over a...
The Spine Journal and the North American Spine Society (NASS) have announced three winners of the 2016 Outstanding Paper Awards at NASS’ 31st Annual Meeting.
These awards recognise excellence in unpublished research in spine care. Winning manuscripts receive a US$10,000...
During the 31st Annual Meeting of the North American Spine Society (NASS), F Todd Wetzel was named NASS president for 2016-17. Wetzel is an orthopaedic surgeon who lives in Wilmington, USA and practices medicine in Philadelphia, USA.
“As a NASS...
According to a new study presented at the 31st Annual Meeting of the North American Spine Society (NASS), high expectations of pain improvement before a common back surgery are actually associated with less pain improvement after surgery.
“While a positive...
Spinal Elements has announced that it will be working with Mighty Oak Medical to market the Firefly surgical guidance system.
The Firefly system consists of bone models and guides precisely matched to the patient’s anatomy through concierge pre-surgical planning and...
Spineology has announced the completion of 100 cases using its new Palisade pedicular fixation system. Palisade is one of several recent additions to Spineology’s Anatomy-Conserving Technology (ACT) product platform.
“Reception of the Palisade system has been outstanding and we expect...
Image-guided surgery systems that provide real-time guidance for minimally invasive spine surgery can help improve patient outcomes, but these systems are costly for many hospitals. Surgeons may rely on checking implant placement with multiple static X-ray images that do...
Pinnacle Spine has announced the launch of its InFill V2 lateral interbody device, which features a larger, single graft chamber and a large load-bearing surface area, which helps restore and maintain disc height and facilitates the formation of a...
Kern Singh, Midwest Orthopaedics at Rush University Medical Center, and his team have started to enrol patients for a clinical study on the FLXfit articulating and lordotic expandable cage (Expanding Orthopedics).
Singh, associate professor, Rush University Medical Center, co-director Minimally Invasive...
DePuy Synthes Spine has announced the launch of the Zero-P Natural plate to help maintain stability and support bone growth in spinal fusion procedures in the neck. The Zero-P Natural plate is designed for use with the CC Natural...
Amniox Medical has announced results of a prospective randomised clinical study of its proprietary cryopreserved Amniotic Membrane as an adjunct to lumbar discectomy. The findings will be presented at the North American Spine Society 2016 annual meeting (26–29 October,...
Life Spine has successfully completed initial cases with its recently-launched Pro-Link Ti stand-alone cervical spacer system. Pro-Link Ti offers a low-profile, stand-alone cervical interbody, incorporating Osseo-Loc, a proprietary surface treatment for titanium which helps create an environment for potential...
The US Food and Drug Administration (FDA) has given clearance to Spineology’s Rampart Duo interbody fusion system. The Rampart Duo device is the first device of its kind to combine PEEK, titanium, and graft containment mesh elements, according to...
Millions of people take opioids for chronic back pain, but many of them get limited relief while experiencing side effects and worrying about the stigma associated with taking them, according to research presented at the Anesthesiology 2016 annual meeting...
Surgery for female adolescent idiopathic scoliosis patients appears to have no impact on complication rates for future pregnancy, according to data presented at the 51st Annual Meeting of the Scoliosis Research Society (SRS; 21–24 September, Prague, Czech Republic).
While those...
An analysis of ≥10 year outcomes data for patients treated surgically for major thoracic adolescent idiopathic scoliosis (AIS) has found that 23% of the surgical outcomes in this cohort were “less than ideal”. The prospective study from the Harms...
Implementing a team-based approach to paediatric spinal surgery “significantly improves surgical and perioperative outcomes”, according to new research presented at the Scoliosis Research Society Annual Meeting (SRS; 21-24 September, Prague, Czech Republic). Even when researchers adjusted their results for...
Beth Israel Deaconess Medical Center in Boston, USA has been added as a clinical site for the INSPIRE study (InVivo Study of Probable Benefit of the Neuro-Spinal Scaffold for Safety and Neurologic Recovery in Subjects with Complete Thoracic AIS...
For patients with chronic back pain, "open" treatment with placebo—informing patients that they are taking an inactive pill, and why it might be helpful—leads to reductions in pain and disability, reports a study published in Pain.
"This study is the...
Titan Spine has doubled its sales management team with the addition of nine new members. According to a company release, this is to meet the growing demand for its line of Endoskeleton products.
Steve Cichy, vice president of Sales for...
The Medicrea Group has received two 510(k) clearances from the US Food and Drug Administration (FDA) for its Pass XS posterior fixation and LigaPass XS band connector components, designed to address paediatric spinal deformities in small stature patients.
The company...
A randomised study has found reduced pre- and postoperative logistics times, as well as a complete elimination of logistics incidents, across 40 surgeries with the use of Safe Orthopaedics’ single-use products.
This 1:1 randomised study was conducted by teams at...
Precision Spine will introduce its MD-Vue lateral access system at the North American Spine Society (NASS) Annual Meeting October 26-29 in Boston, USA. According to a company release, the system was designed in collaboration with prominent lateral approach spinal...
The Foothills Medical Centre in Calgary has been added as a Canadian clinical site for InVivo Therapeutics’ INSPIRE study (InVivo Study of Probable Benefit of the Neuro-Spinal Scaffold for Safety and Neurologic Recovery in Subjects with Complete Thoracic AIS...
SpineGuard is to launch its next-generation PediGuard “Threaded” device using the company’s Dynamic Surgical Guidance technology the 31st annual meeting of the North American Spine Society (NASS) in Boston, USA, October 26-29 2016.
The PediGuard Threaded device with DSG is...
Spineology has obtained US Food and Drug Administration (FDA)-clearance for the use of allograft bone with its Rampart Interbody Fusion Devices.
“This approval and our recent partnership expansion with Musculoskeletal Transplant Foundation (MTF) provides us the ability to pair MTF...
Researchers from the Ohio State University (Columbus, USA) have discovered that spinal cord injury alters the type of bacteria living in the gut, and that these changes can exacerbate the extent of neurological damage and impair recovery of function....
Zyga Technology has released 12-month fusion and clinical results for patients receiving SImmetry sacroiliac joint fusion with decortication. Study results were presented at the 2016 Society for Minimally Invasive Spine Surgery (SMISS) annual meeting (13-15 October 2016) by William...
Norgine Ventures BV has entered into a definitive agreement to acquire SpineVision SA, an integrated spinal technology company focused on the development and marketing of implants and instrumentation for spinal treatment.
Arnaud Brisard, chief executive officer, SpineVision comments, “I am...
Mazor Robotics has announced that it received purchase orders for 25 systems during the third financial quarter of 2016, including pre-launch orders for the recently unveiled Mazor X, a transformative guidance platform for spinal surgeries. The Mazor X will...
ChoiceSpine has announced the appointment of Christy Cote as vice president of Biologics. According to a company release, she is a business leader with over 14 years’ experience in stem cell technology, surgical implants, and regenerative therapies.
Prior to joining...
SI-Bone has submitted INSITE (Investigation of Sacroiliac Fusion Treatment) two-year randomised controlled trial data to Yale University's Open Data Access (YODA) programme.
INSITE is a randomised controlled trial of minimally invasive sacroiliac joint fusion using the company’s iFuse devices, in...
The Charité–Universitätsmedizin hospital in Berlin, Germany has started to provide patient exams with its new EOS imaging system. Charité is a leading hospital in Germany with 17 different Charité centres in the Berlin area.
Established over 300 years ago, Charité...
Bone Therapeutics has reported positive efficacy data for its phase IIA spinal fusion trial with Allob. Results of the first half of patients in the study show evidence of successful fusion and important clinical improvements in function, pain and...
Alphatec Holdings, the parent company of Alphatec Spine, has announced a reduction in the company's workforce and changes to the company's executive leadership team.
Michael O'Neill, Alphatec's chief financial officer and treasurer, has resigned effective October 5, 2016. Dennis Nelson,...
Stryker’s Spine division has announced the launch of its Lite Bio delivery system, a hand-held device used to facilitate delivery of bone graft material to spinal surgery sites.
It is designed to simplify graft delivery, accommodate a surgeon’s preferred...
K2M has received 510(k) clearance from the US Food and Drug Administration (FDA) to expand its Cascadia lateral interbody system, featuring the company’s Lamellar 3D titanium technology.
K2M’s Lamellar 3D titanium technology uses a 3D printing method to create structures...
Titan Spine has expanded the distribution of its line of Endoskeleton titanium implants featuring the company’s new proprietary NanoLock surface technology to all of the USA. The launch follows the alpha introduction of the NanoLock technology initiated recently in a...
The majority of US National Football League (NFL) players who undergo surgery for a herniated disc in the cervical spine are able to resume their careers and perform at a high level, suggests a study in Spine.
Even players with...
US district court judge, Timothy S Black (Southern Ohio, USA) has dismissed the accusation of several hundred plaintiffs that they have been treated off-label with Medtronic’s Infuse.
The plaintiffs were all former patients of Atiq Durrani (Cincinnati, USA), an orthopaedic...
Identifying frailty in older patients could increase their chances of surviving surgery, as well as improve their overall outcomes, according to a new study posted online in The Annals of Thoracic Surgery.
“Patients with frail health have less ability to...
Studies have shown that activity-based interventions can offer a promising approach to the improvement of motor function following spinal cord injury. Sunil Agrawal, professor of mechanical engineering and of rehabilitation and regenerative medicine at Columbia Engineering (New York City,...
A Dutch cost-utility study published in The Spine Journal argues that implantation of interspinous process devices as an indirect decompressing measure is “highly unlikely to be cost-effective compared with bony decompression for patients with intermittent neurogenic claudication caused by...
Rafael De la Garza-Ramos (Monterrey, Mexico, currently resident at Montefiore Medical Center, Bronx, USA) and others report in the International Journal of Spine Surgery that vertebral augmentation, either through vertebroplasty or kyphoplasty, is associated with a significant reduction in...
Early bird registration for NSpine 2017 has opened, offering discounted registration until 30th November.
NSpine is collaborating with the British Scoliosis Research Foundation and NuVasive to offer registration to the 15th International Philipp Zorab Symposium at a reduced rate.
The collaboration...
The first subject has been enrolled in the ReActiv8-B Clinical Trial of the Mainstay Medical ReActiv8 device. The trial is intended to gather data in support of an application for pre-market approval from the US Food and Drug Administration...
The ApiFix system has now been used to correct scoliosis in 100 patients with a first case at the Paul Gerhardt Diakonie hospital in Berlin, Germany. The 100th case was Miguel Alquiza's first time implanting the ApiFix system. According...
Mainstay Medical has announced the one-year results from the ReActiv8-A Clinical Trial, an international, multi-centre, prospective, single arm trial for ReActiv8 in people with disabling chronic low back pain and few other treatment options.
The one-year results show sustained performance...
Spineology has expanded its relationship with Musculoskeletal Transplant Foundation (MTF), the USA’s leading tissue bank. MTF will now be the sole tissue provider for Spineology’s allograft product lines, including the newly launched Incite cortical fibres, a unique and versatile...
Ulrich medical has announced the US market release of its uCerv titanium spinal implants as an adjunct to its current uCerv PEEK Optima interbody system, which is an existing product used for anterior cervical discectomy and fusion surgical procedures.
"Spinal...
Company: XYZ Medical
Position: Marketing Manager
Contact: [email protected]
Application deadline: 26 September 2017
Job description:
The marketing manager manages the day to day marketing activities of the organisation and long term marketing strategy for the company.
Duties of the Marketing Manager include:
Managing all marketing...
Band-Lok has announced the performance of the first spinal surgery using the company’s pedicle-sparing polyester band technology.
Mike Albert performed the first case on August 22, 2016, at Dayton Children’s Hospital in Dayton, Ohio. “The technology’s unique tether clamp system...
NuVasive has received 510(k) clearance from the US Food and Drug Administration (FDA) for the company’s Magec system to be surgically implanted using its Reline posterior fixation system for treating patients with severe spinal deformity conditions.
According to a company...
Life Spine has announced that the US Food and Drug Administration (FDA) has given 510(k) marketing clearance to the company’s Pro-Link Ti stand-alone cervical spacer system. The system features the company’s Osseo-Loc technology.
“Osseo-Loc is a proprietary surface treatment for...
A small study from Inspired Spine has found a significant reduction in back pain following minimally invasive direct thoracic interbody fusion (MIS-DTIF).
The study included four participants and measured patient-reported pain, surgery time and complications.
Before surgery, patients reported an average...
K2M has received 510(k) clearance from the US Food and Drug Administration (FDA) for screw and connector components for its Mesa spinal system. This clearance enables these screw and connector components to be used as a part of a...
Tuberculous spinal epidural abscess is an uncommon pathology that needs an urgent intervention to decompress pressure on the spinal epidural sac, cord and roots, writes Ghazwan A Hasan.
Vertebral tuberculosis accounts for less than 1% of all tuberculosis infections. It...
A study from NSpine has shown that a majority of women experience problems in sexual function following anterior spinal surgery. The study, which was presented at Spineweek (Singapore; 16-20 May 2016) by Irene Hernandez-Sanchez (Birmingham, UK) during the “All...
Implanet has announced successful results for the first surgical procedures using the Jazz Lock.
Having obtained CE marking and 510K clearance, Jazz Lock has been used in select hospitals in France, Italy and the USA. According to a press release,...
EOS imaging has sold an EOS system to the Carolinas HealthCare System (Charlotte, USA).
The system will be located at a dedicated imaging centre in Carolina HealthCare’s Morehead Medical Plaza campus in Charlotte. According to a company release, it will...
Christopher Bono’s interest in surgery was piqued after watching live operations on television as a teenager. After training and working in New York, USA, Bono became involved in orthopaedic surgery and spinal research. During his career he has worked...
Vexim has signed an agreement with Creatori Health for the distribution of its portfolio in South Africa.
Vexim’s portfolio gained reimbursement from one of the leading medical health insurance in South Africa.
“With the support of Vexim, and the granted reimbursement...
Highlights:- Risk stratification is not a “universal language”, numerous IMAST presentations find - Posterior BMP-2 use does not lead to increased cervical deformity surgery-related complications - UHMWPE cable feature - Profile: Christopher Bono
https://spinalnewsinternational.com/wp-content/uploads/sites/11/2016/09/40-Spinal-News-low-res3.pdf
Chemists from Trinity College Dublin, (Dublin, Ireland) in collaboration with the Royal College of Surgeons in Ireland (RSCI), have devised a new scanning technique that can produce extremely high-resolution 3D images of bones without exposing patients to X-ray radiation.
The...
A large Japanese study has found that a significant number of spinal stenosis patients using health insurance claims are elderly people (>80 years), with most receiving non-surgical treatment. The research was published in Spine.
Lead author Izumi Kuboyama, The University...
Alphatec Holdings, the parent company of Alphatec Spine, has completed the sale of its international operations and distribution channel to Globus Medical. With the closing of the transaction, Alphatec is now focused solely on the US market.
The company has...
Thomas J Hwang (Harvard University, Cambridge, USA, Brigham and Women’s Hospital and Harvard Medical School, Boston, USA) and others report in the British Medical Journal that medical devices that are approved in the European Union (EU) prior to being...
The use of computers to map the spine, to plan surgery, and to aid in the manufacturing of medical devices is becoming increasingly common in spinal surgery. Spinal News International speaks with Andrew G King about his experience with patient-specific rods (UNiD,...
A team of doctors from Keck Medical Center of the University of Sourthern California (Los Angeles, USA) have become the first in the US state of California to inject an experimental treatment made from stem cells, AST-OPC1, into the damaged...
Flakes of graphene welded together into solid materials may be suitable for bone implants, according to a study led by Rice University (Houston, USA) scientists.
The Rice lab of materials scientist Pulickel Ajayan and colleagues in Texas, Brazil and India...
According to new research from the Hospital for Special Surgery in New York City, USA, 36.5% of patients undergoing surgical treatment for adult spinal deformity in the US Nationwide Inpatient Sample (NIS) has been coded with at least one...
Vexim has received regulatory approval from the Therapeutic Goods Administration to commercialise its SpineJack and the cement delivery system, Masterflow, in Australia.
According to a press release, the company is expanding its international presence whilst preparing the development of new...
Today, transcutaneous spinal fixation is efficient in terms of solidity and reduction. Achieving real fusion remains a problem, however, as does the successful navigation of each surgical approach. Fusion takes place, after all, at the hardest point to reach...
Whilst a number of studies have assessed the incidence of complications in association with posterior BMP-2 (bone morphogenetic protein-2) use, researchers from the International Spine Study Group noted that none focus on cervical deformity. Presented by Han Jo Kim...
On 17 August, a multicentre, randomised, double-blind, placebo-controlled trial—the VAPOUR trial—was published online ahead of print in The Lancet. Six-month data from the trial provide the first sham-controlled evidence in support of using the procedure.
Data from VAPOUR (Safety and...
The variety of procedures in spinal surgery is extensive. While anterior and posterior open approaches are the conventional approaches, it is the posterior approach that is most frequently used. Traditionally, the open posterior exposure involves a central midline incision...
Minimally invasive approaches to spinal surgery can lead to less blood loss, faster rehabilitation and a reduction in patient scarring. However, these procedures may also lead to increased procedure times, higher doses of radiation and less surgical precision. The Artis zeego fixed...
AESCULAP®’s Plasmapore® titanium coating has evolved over the past thirty years. From its 1986 commercial introduction on the BICONTACT hip implant system to its 1995 application to the PROSPACE® posterior lumbar interbody fusion system, the surface coating has provided an ideal environment for...
This year’s Spineweek meeting (Singapore; 16–20 May) featured a number of presentations from F-MARC, the Fédération Internationale de Football Association (FIFA) Medical Assessment and Research Centre. Among the data presented at the meeting, one first-of-its-kind study found a significantly...
A study using US insurance data has shown the risk of complication and the incidence of novel lumbar pathology following minimally invasive sacroiliac joint fusion surgery to be higher than previously reported in the literature. This respective study—currently in...
The US Patent and Trademark Office (USPTO) has granted a patent for the Jazz platform to Implanet. This protects the company’s intellectual property until 2032.
The Jaz platform’s intellectual protection now covers the braided implant and its tensioning system, the...
Initial cases have been successfully completed with Life Spine’s Sentry lateral plate system. The product is designed to offer a low profile, intuitive design with a cam-locking mechanism, intended to provide visual, tactile and audible confirmation of final locking.
“The...
OrthoPediatrics has launched its new BandLoc 5.5/6mm system, which has been both CE-marked and US Food and Drug Administration-approved.
BandLoc is designed to offer a pedicle-sparing, band passage technique for treating a wide variety of complex spinal pathologies, including scoliosis....
Tyber Medical has launched their lateral plating system commercially.
Through the use of new and patented features, the implant is designed to provide three points of fixation in each vertebral body, eliminating some of the instrumentation required by other systems,...
Spinal Simplicity has launched a new generation of Minuteman G3 fusion device, featuring a US Food and Drug Administration (FDA)-cleared coating of hydroxyapatite.
"Hydroxyapatite has been used in orthopaedic procedures for the last 30 years because of its successful track...
SeaSpine has entered into a definitive agreement to acquire all assets of NLT Spine, an Israel-based medical device company developing spinal products for minimally invasive surgery.
NLT’s platforms include vertical, lordotic and footprint expanding interbody technologies for use in lumbar...
CTL Medical has established a General Services Administration (GSA) partnership with Firehouse Medical to sell its line of innovative spinal implants and devices to the US government. GSA contracts allow commercial companies to establish long-term, government-wide contracts and sell...
The ninth spinal cord injury patient in the INSPIRE trial—implanted with InVivo Therapeutics’ neuro-spinal scaffold last month—is showing signs of improvement. The patient is the fifth of eight patients in the company’s study to show an American Spinal Injury...
Medtronic has completed the second tranche of a previously agreed equity investment in Mazor Robotics.
According to a press release, the triggering milestone for this second tranche investment was the July 12, 2016 unveiling by the company of Mazor X.
“Since...
Medicrea has appointed Richard Kienzle as chief commercial officer and business development officer. Kienzle will also join Medicrea’s board of directors. Kienzle is best known as a founding member of Globus Medical.
“I am…excited to welcome Rick Kienzle to the...
The US Food and Drug Administration (FDA) has cleared an expanded range of Valeo II lateral lumbar sizes (Amedica). The additional sizes of the Valeo II LL interbody fusion device will be commercially available August 29, 2016.
The Valeo II...
NovaBone has received the Brazilian ANVISA Good Manufacturing Practices (GMP) medical device certificate.
ANVISA is Brazil's equivalent to the US Food and Drug Administration (FDA), and is responsible for the oversight of all medical device and pharmaceuticals in the Brazilian...
As part of an ongoing collaboration between Rensselaer Polytechnic Institute (Troy, USA) and the Icahn School of Medicine at Mount Sinai (New York City, USA)—a partnership that draws upon the expertise of both schools to address significant health problems—researchers...
SI-Bone has announced the publication of two-year results from INSITE (Investigation of Sacroiliac Fusion Treatment) a landmark prospective, multicentre, randomised controlled trial of minimally invasive sacroiliac joint fusion with iFuse compared to non-surgical management. Results of this level 1...
Titan Spine has expanded its distribution agreement with strategic partner MBA to provide its line of Endoskeleton titanium implants to practicing spinal surgeons in Italy.
Under the agreement, initially announced in September 2015, Titan Spine provides its spinal interbody fusion...