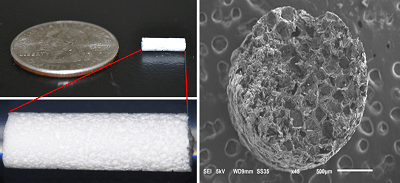
InVivo Therapeutics has announced that a new patient has been enrolled into the INSPIRE study (InVivo Study of Probable Benefit of the Neuro-Spinal Scaffold for Safety and Neurologic Recovery in Subjects with Complete Thoracic AIS A Spinal Cord Injury) at Oregon Health & Science University (OHSU) in Portland, USA.
Ahmed M Raslan, and Jason J Chang, assistant professors of Neurological Surgery and study investigators, performed surgery and the implantation on the T9 neurologically complete patient approximately 36 hours after the injury occurred.
Mark Perrin, InVivo’s Chief Executive Officer and Chairman, says, “We are pleased that the patient is doing well and wish them continued recovery. We have nine patients enrolled and in follow up and, because we now have over 25 INSPIRE sites open, expect enrolment to increase in the coming months.”
InVivo Therapeutics has also added the University of Iowa Hospitals and Clinics in Iowa City, USA, and Allegheny General Hospital, Pittsburgh, USA, as clinical sites for the INSPIRE study.
University of Iowa Hospitals and Clinics is a 732-bed hospital which, in fiscal year 2015, received more than 57,000 emergency room visits.
“Two active areas of spinal cord injury research involve timing and the use of biomaterials. The INSPIRE study represents the marriage of these two concepts and we look forward to participating,” says Patrick Hitchon, professor in the departments of Neurosurgery and Biomedical Engineering and Principal Investigator at University of Iowa Hospitals and Clinics. Saul Wilson and Chandan Reddy are co-investigators for the research trial.
Allegheny General Hospital, which treats more than 24,000 inpatients each year, was the first hospital in the region to receive designation as a Level I Shock Trauma Center. This is the highest designation available.
“My research interests lie at the intersection of neuro-oncology and spinal disorders. While we routinely enter the spinal cord to remove tumours, the INSPIRE study is the first opportunity that affords us the ability to enter the spinal cord in trauma cases. I look forward to being a part of this study, which has the potential to change the standard of care,” says Terrence Julien, system director of Surgical Neuro-Oncology, system co-director of Minimally Invasive Spine Surgery, and principal investigator at the study site.
There are now 27 clinical sites participating in the clinical study:
Allegheny General Hospital, Pittsburgh, USA
Banner University Medical Center, Tucson, USA
Barnes-Jewish Hospital at Washington University Medical Center, St Louis, USA
Barrow Neurological Institute–St. Joseph’s Hospital and Medical Center, Phoenix, USA
Ben Taub Hospital/Baylor College of Medicine, Houston, USA
Beth Israel Deaconess Medical Center, Boston, USA
Carolina Neurosurgery and Spine Associates/Carolinas Rehabilitation, Charlotte, USA
Cooper Neurological Institute, Camden, USA
Foothills Medical Centre, Calgary, Canada
Goodman Campbell Brain and Spine/Indiana University Health Neuroscience Center, Indianapolis, USA
Hospital of the University of Pennsylvania, Philadelphia, USA
Keck Hospital of University of Southern California, Los Angeles, USA
Medical College of Wisconsin/Froedtert Hospital, Milwaukee, USA
Mount Sinai Hospital, New York, USA
Northwestern Medicine, Chicago, USA
Oregon Health & Science University, Portland, USA
Rutgers New Jersey Medical School, Newark, USA
Thomas Jefferson University Hospital, Philadelphia, USA
Toronto Western Hospital, Toronto, Canada
University of California, Davis Medical Center, Sacramento, USA
University of California, San Diego Medical Center, San Diego, USA
University of Iowa Hospitals and Clinics, Iowa City, USA
University of Kansas Medical Center, Kansas City, USA
University of Louisville Hospital, Louisville, USA
University of Pittsburgh Medical Center Presbyterian, Pittsburgh, USA
University of Virginia Health System, Charlottesville, USA
Vidant Medical Center, Greenville, USA













