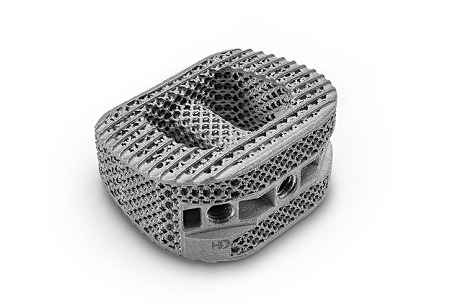
The US Food and Drug Administration (FDA) has cleared HD Lifesciences’ NanoHive interbodies. The company released the devices to the US market last month.
Each interbody device delivers “four clinical improvements,” compared to other comparable devices, according to Lucas Diehl, chief executive officer of HD LifeSciences.
Diehl states, “Our NanoHive interbodies deliver…stiffness, ingrowth, ongrowth, and imaging. Our customers no longer need to compromise on imaging or stiffness to use a biologically active interbody, and vice versa. Feedback has been overwhelmingly positive.”
HD LifeSciences’ newly-approved devices are based on their proprietary Soft Titanium technology platform. The implants feature a honeycomb lattice.
Created through high-definition additive manufacturing, one device is designed for anterior lumbar interbody fusion (ALIF), one for posterior lumbar interbody fusion (PLIF), and one for transforaminal lumbar interbody fusion (TLIF).