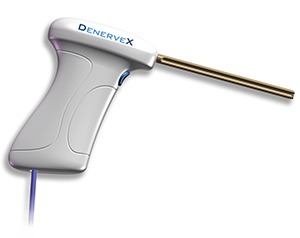
Medovex has received CE mark approval for the Denervex system allowing the company to market the Denervex system in Europe.
The Denervex system is designed to denervate and removes capsular tissue from the facet joint in one single procedure. Treatment results from the combined effect of a deburring or polishing action and radiofrequency ablation treatment on the facet joint. Using this new technique, the slowly rotating burr removes the targeted facet joint synovial membrane and joint surface while the heat ablation destroys tissue and denudes any residual nervous and synovial membrane overlying the joint, effectively removing the end point sensory tissue of the joint.
Jarrett Gorlin, Medovex chief executive officer, comments, “Design, development and commercialisation of our flagship patented Denervex system has been years in the making. It was designed by surgeons for surgeons to be less invasive with faster recovery time than current surgical treatment options, and is designed to provide for a longer lasting treatment solution while offering potential savings to the health care system.”
The Denervex system consists of the Denervex device kit, containing a single use medical device and the Denervex Pro-40 power generator.













