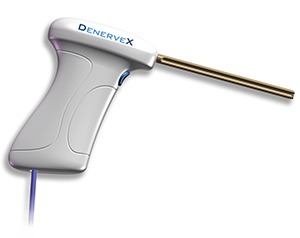
Medovex, a developer of medical technology products, has entered into a partnership with Technology Consult Berlin (TCB) for distribution of its Denervex system throughout Germany. TCB is expected to provide sales, marketing and distribution services.
TCB—an associated partner of Kalms & Partner Consulting based in Berlin, Germany—offers a consultancy service for medical device manufacturers and selected services for pharmaceutical companies, includong sales and marketing, full distribution services, strategic and operational support in the fields of market access, health economics, reimbursement and business development.
The company previously announced that the reimbursement authority in Germany had renewed reimbursement payment coding for the DenerveX system technology for 2017.
The renewed reimbursement coding, effective immediately, was released in the Diagnosis-Related Group (DRG) system in 2017 in Germany. This new coding allows for hospitals and outpatient centers to receive reimbursement for the use of the system for the treatment of facet joint syndrome in the spine.
The DenerveX system is not yet commercially available in the EU and the US. The system is designed to provide longer lasting relief of pain associated with the facet joint. It consists of the DenerveX device kit, containing a single use device, and the DenerveX Pro-40 power generator. The DenerveX system is designed to provide a minimally invasive treatment option which combines two actions into one device.













