This article is sponsored by Cerapedics, Inc.
A FAST, RELIABLE AND PROVEN ALTERNATIVE TO AUTOGRAFT
i-FACTOR peptide-enhanced bone graft is challenging the gold standard in bone grafting and revolutionising expectations for spinal fusion.
Bone graft substitutes are used extensively in spinal surgery as a means to encourage bone healing and fusion. Today’s market is flooded with a variety of different products, all aiming to replicate the performance and safety of autograft bone which clinicians still regard as the “gold standard”. Amongst the many bone graft substitutes available, i-FACTOR Bone Graft (Cerapedics, Inc.) is the only synthetic bone graft to demonstrate superiority to autograft according to independent radiographic evidence and patient-reported outcomes from a prospective, randomised Level I clinical study. i-FACTOR Bone Graft offers a powerful and safe mechanism of action, proven by the highest quality of evidence which is demanded by surgeons, administrators and payers to support therapeutic and coverage decisions.

Autograft bone has long been considered the gold standard for spinal fusion as it has osteoconductive, osteoinductive and osteogenic properties. However, its use is often associated with donor site morbidity, and does not offer reliable levels of quality or volume. This has stimulated the search for substitute biomaterials that offer an equivalent level of performance.
Whether natural (allograft, simple demineralised bone matrix [DBM]) or synthetic (hydroxyapatite, calcium phosphate), the majority of materials are simple osteoconductive substitutes; offering a scaffold for fusion that is gradually resorbed over time, but does not actively promote bone growth.
In recent years, a number of products have been introduced that incorporate an active component to positively influence the fusion process. Biologic bone grafts are often classified as drug/device combinations, of which recombinant human bone morphogenetic proteins (rhBMPs) are the primary example. These potent exogenous growth factors stimulate stem cells to differentiate down the osteogenic lineage, but the enhanced fusion potential comes with high costs and the risk of increased complication rates.
Unlike other biologic bone grafts, i-FACTOR Bone Graft has a unique and powerful mechanism of action that is based upon the biological activity of a 15-amino acid peptide sequence naturally found in human Type I collagen. This protein segment is responsible for the attachment and proliferation of osteogenic cells that produce bone. i-FACTOR Bone Graft combines a synthetic version of this peptide, known as P-15, with a hydroxyapatite, anorganic bone mineral (ABM). This combination provides a proven osteoconductive scaffold with an abundance of P-15 attachment sites, allowing for increased cell binding at the fusion site. Cells have a naturally high affinity for P-15, and once attached, are activated to begin the production of essential growth factors. This surface bound method of action reduces the risk of bone formation away from the graft site, and ensures growth factors are produced and regulated by the cells in physiological concentrations. The P-15 within i-FACTOR Bone Graft amplifies the natural bone healing process resulting in rapid bone formation that is safe and predictable.
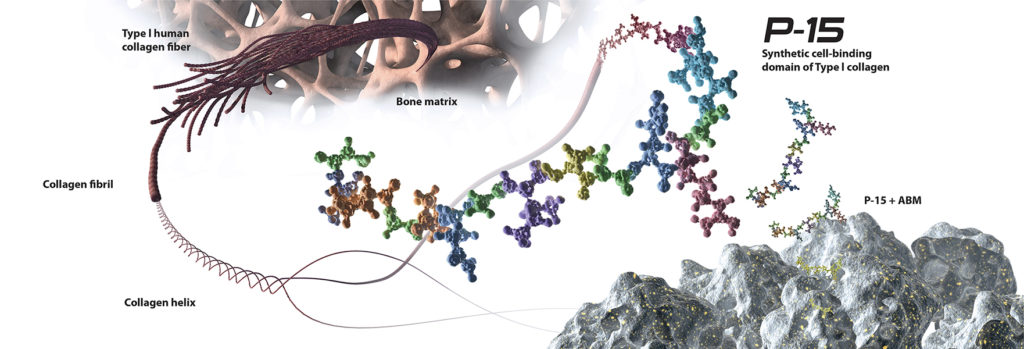
i-FACTOR Peptide Enhanced Bone Graft is a clinically proven, biologic bone graft that was developed to be an alternative to expensive engineered growth factors (rhBMPs) with documented safety issues, and experimental cell-based allografts with no objective data. Recently-published Level I evidence has demonstrated that i-FACTOR Bone Graft yields significantly superior overall outcomes over the gold standard; autograft bone.1 Radiographic evidence has also shown i-FACTOR Bone Graft to be statistically superior to autologous bone in facilitating the formation of bridging bone in posterior lumbar interbody fusion.2 With P-15 as the active component, i-FACTOR Peptide Enhanced Bone Graft is challenging the gold standard in bone grafting and revolutionising expectations for spinal fusion.
In the United States, i-FACTOR™ Peptide Enhanced Bone Graft – Putty is indicated for use in skeletally mature patients for reconstruction of a degenerated cervical disc at one level from C3-C4 to C6-C7 following single-level discectomy for intractable radiculopathy (arm pain and/or a neurological deficit), with or without neck pain, or myelopathy due to a single-level abnormality localised to the disc space, and corresponding to at least one of the following conditions confirmed by radiographic imaging (CT, MRI, X-rays): herniated nucleus pulposus, spondylosis (defined by the presence of osteophytes), and/or visible loss of disc height as compared to adjacent levels, after failure of at least 6 weeks of conservative treatment. i-FACTOR™ Peptide Enhanced Bone Graft must be used inside an allograft bone ring and with supplemental anterior plate fixation.
CAUTION: i-FACTOR™ Flex FR is not available for sale in the United States.

Evidence based spinal biologics: Efficacy of i-FACTOR bone graft vs. autograft in anterior cervical discectomy and fusion.
The application of biologics in spine surgery allows the potential to significantly influence surgical success with improved fusion rates, pain reduction, and clinical outcomes. Although clinicians still consider autograft as the gold standard for fusion, in practice, a variety of other bone graft options are being used with increasing regularity, either as a means to extend the autograft volume or as a substitute. Choosing the right bone graft option should be based upon clinical factors, including an understanding of the biology of the healing environment, together with a critical review of the supporting scientific and clinical evidence. Unfortunately, there is a paucity of high quality evidence supporting most bone graft options.
I strongly believe that clinical decisions should be made on a solid foundation of evidence, and I am proud to have participated in a prospective, randomised, multicentre FDA IDE evaluation of a novel bone biologic—i-FACTOR Peptide Enhanced Bone Graft—for use in cervical interbody fusion.
i-FACTOR Bone Graft is a composite bone substitute material consisting of a synthetic cell-binding domain of Type I human collagen (P-15) adsorbed onto anorganic bone mineral. P-15 has demonstrated bone-healing efficacy in dental, orthopaedic and non-human applications. We assessed the safety and efficacy of i-FACTOR Bone Graft relative to autograft in single-level anterior cervical discectomy and fusion (ACDF) for cervical radiculopathy.
Patients were randomised to receive either autograft (n=154) or i-FACTOR Bone Graft (n=165) in a cortical ring allograft. Study success was defined as noninferiority in fusion, Neck Disability Index (NDI) and neurological success endpoints, and similar adverse events profile at 12 months. With a follow-up rate of 87% at 12 months, the results of the clinical trial demonstrate that i-FACTOR Bone Graft is a safe and effective treatment for symptomatic radiculopathy due to single-level cervical disc disease. Both i-FACTOR Bone Graft and autograft subjects demonstrated a high fusion rate (88.97% and 85.82% respectively), significant improvements in NDI (28.75 and 27.40 respectively), and high neurological success rate (93.71% and 93.01% respectively). Additionally, there was no difference in the rate of adverse events (83.64% and 82.47% for i-FACTOR Bone Graft and autograft respectively).
Of note, a composite endpoint consisting of fusion, NDI, neurological success and safety success was included in the primary data analysis. In this analysis, i-FACTOR Bone Graft patients demonstrated an overall success rate that was statistically superior to the autograft control group (68.75% vs 56.94% respectively, p=0.0382).
In this FDA IDE pivotal clinical study, i-FACTOR Bone Graft reached all four FDA-mandated success criteria for use in ACDF relative to the autograft control. These 12-month findings, published in Spine, corroborate the positive bone healing influence of P-15 found in the literature.1 This study provides Level I evidence that the use of i-FACTOR Bone Graft in an interbody environment is safe, effective and produces results that are similar to and, for some metrics, superior to autograft bone.
1. PM Arnold, RC Sasso, ME Janssen, et al. Efficacy of i-Factor Bone Graft versus autograft in Anterior Cervical Discectomy and Fusion: Results of the Prospective, Randomized, Single-blinded Food and Drug Administration Investigational Device Exemption Study. Spine 2016; 41(13):1075–83.
2. P Lauweryns P, Y Raskin. Prospective analysis of a new bone graft in lumbar interbody fusion: Results of a 2-year prospective clinical and radiological study. Int J Spine Surg 2015; 9.

Robust evidence. Broad applications.
The biologics market is saturated with a host of different bone graft substitute options. Why did you choose i-FACTOR?
After canvassing the opinions of my colleagues on our current use of biologics, I found no real consensus as to why we were using particular bone grafts. I realised that, as a department, we needed to understand the evidence to support a particular bone graft’s adoption.
i-FACTOR was the only bone graft substitute that was able to demonstrate robust scientific and clinical evidence. From the peer reviewed publications, our department understood that i-FACTOR would be a product that would work, not only in conjunction with autologous bone or bone marrow aspirate, but most importantly as a standalone bone graft substitute.
i-FACTOR is the only peptide-enhanced bone graft. How does it work and what do you see as its main clinical benefits?
The peptide (P-15) contained in i-FACTOR is recognised as a binding site for osteogenic cells. i-FACTOR attracts the cells and enhances their activity to get them forming bone quickly and safely. Evidence for a lot of the other products is lacking. The studies Cerapedics have produced, however, demonstrate fusion in the interbody space. I am convinced by the data out there that it will do what it says on the tin—that it will get patients to fuse. I am hoping to work closely with Cerapedics to look at my centre’s short- and long-term follow-up data in more detail.
In what type of procedures do you normally use i-FACTOR?
I use i-FACTOR whenever I need a fusion, but have a limited supply of autologous bone. This means I use i-FACTOR in pretty much every patient over 65—or where there are comorbidities at play—whether I am doing an anterior interbody fusion, a posterior interbody fusion or a posterolateral fusion. I rely on i-FACTOR to give me a fast and good quality fusion which, ultimately, benefits my patients.
Outside of the USA, i-FACTOR is available in two product forms (Putty and Flex FR), how do these fit with your normal treatment algorithm?
When I was first introduced to i-FACTOR, I predominantly used the putty, which is very easy to handle. I use a 3D-printed porous titanium cage for posterior cases, where I impact the putty into the pores to try and stimulate the initiation of fusion onto the cage.
When I need pack anterior lumbar cages I use i-FACTOR Flex FR cut to size. I also use i-FACTOR Flex FR in my posterolateral cases and place the strips down each gutter onto a bed of whatever autograft I have available. If I have enough of the patient’s own bone then I tend to mix 30% to 50% i-FACTOR Putty with the autograft to enhance the fusion potential.
Generally speaking, do you see i-FACTOR Bone Graft as a suitable alternative to rhBMP-2 in cases where patient safety is a concern?
The safety profile of i-FACTOR is very good, with little or none of the soft tissue reaction you get with rhBMP-2. My experience with rhBMP-2 is in complex revision surgery, where I have not been convinced that it delivers on its promises. As well as this, rhBMP-2 is phenomenally expensive. The evidence shows that i-FACTOR works as a viable alternative to rhBMP-2, without the expense and the complications.

Active. Safe. Efficacious.
What are your expectations for a bone graft substitute in day-to-day clinical practice?
Every bone graft substitute has to be measured against our gold standard—autograft. When we need an alternative, it has to be measured against autograft in terms of efficacy and safety, and its results have to be proven by scientific evidence.
Do all bone graft substitutes meet these expectations?
In terms of synthetics, we have products that will meet some of those criteria, but not all of them. Biologics and growth factors such as rhBMP-2 can certainly work well, but the safety profile is very poor. This is especially true in the cervical spine where the FDA warns against their use in this area because of the risk of potentially fatal complications.
In today’s environment, cost is also a major factor. Several biologic products are prohibitively expensive for use in routine applications.
Some alternatives have reasonable safety profiles and great marketing claims, but they do not have much in the way of evidence. Stem cell-based bone substitutes, which are marketed as safer than biologics and growth factors, do not have much in the way of clinical data to show they work in a real-life human application.
What makes i-FACTOR different, and why is it now the product of choice for your clinical practice?
I strongly believe in the use of synthetic materials in spinal surgery. Although there are lots of different bone graft substitutes and synthetic bone grafts on the market, i-FACTOR is different. It is the only one which is peptide-enhanced. It actually has an active component to it, rather than just being a scaffold for bone formation. The use of peptide means that bone will only form where you want it to. Compared to others where the biologic agent infuses into the surrounding tissue, we do not see uncontrolled bone growth with i-FACTOR.
As a clinician, what takeaways from i-FACTOR’s multicentre Level 1 FDA study did you find most impactful?
Firstly, this the only FDA study of a bone graft substitute specifically for use in the cervical spine—cervical applications for other bone grafts are all off-label. I use i-FACTOR in anterior cervical discectomy as it is the only bone graft substitute that has been shown to be safe and efficacious in that situation. Secondly, not only was i-FACTOR shown to be as safe as autograft, but its clinical efficacy was actually superior to autograft. That is unique on the market.
To what extent did pre-market approval (PMA) and Level I evidence influence your decision to adopt i-FACTOR?
The hope is that insurance payers will recognise this cervical indication. Right now, most bone grafts we are not reimbursed by insurance carriers; we are just using them because they work. I hope that PMA approval will improve the cost profile for hospitals.
How has i-FACTOR improved your clinical practice?
I have been using synthetics in the cervical spine for about 10 years. When I started using i-FACTOR I thought it would be as good as the synthetics I was already using. However, I have been amazed at how quickly I am now seeing fusion occur. Even the devout believer in synthetic bone grafts in me was surprised by how well this product works.
With prior synthetics, I would see fusion occurring in the cervical spine at approximately six months, I am now seeing those same radiographic results at three months. The earlier the spine fuses, the better its overall stability. i-FACTOR is increasing my fusion rates, both in percentage of fusion and also the speed of fusion.















Can I-Factor leak into other tissue not intended for bone graft. And if so what are the concerns or potential risks.