
The first implantation of Camber Spine Technology’s Enza minimally invasive zero-profile anterior lumbar interbody fusion (ALIF) device in a two-level lumbar procedure at levels L4/L5 and L5/S1 has taken place.

The procedure was performed at Kennedy University Hospital (Washington Township, USA) by Jeffrey Gleimer.
Enza is the company’s first device launched with integrated fixation to promote minimally invasive mechanical fusion.
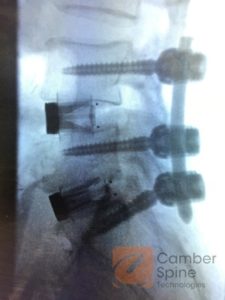
“Today I implanted the Enza ALIF device in a patient to replace two damaged discs. The design of the implant has perfected true direct midline fixation within the retroperitoneal space for lumbar fusion… providing a simple, easy-to-implant device with superior osseous fixation, even in significantly sclerotic bone, while also significantly reducing my operative time,” commented Gleimer.
The device is indicated for use with autogenous bone graft in patients with degenerative disc disease (DDD) at one or two contiguous levels from L2 to S1. These implants may be implanted via laparascopic or an open anterior approach.













