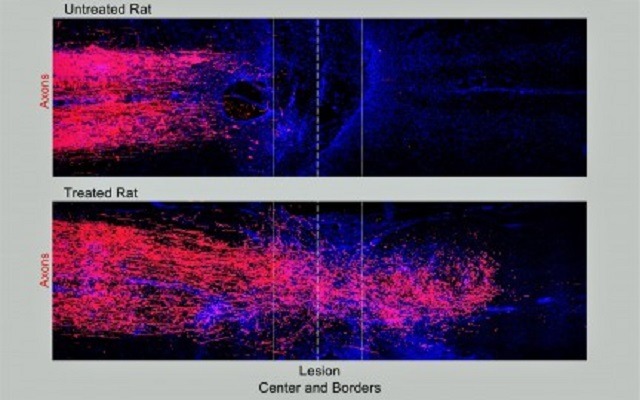
At top, damaged axons in an untreated rat stop at the border of the spinal cord injury. Below, axons in a treated rat regrew across the scar, creating new connections on the other side.
Neuroscientists at UCLA, Harvard University and the Swiss Federal Institute of Technology have identified a three-pronged treatment that triggers axons to regrow after complete spinal cord injury in rodents. In addition to facilitating axon growth through scar tissue, the treatment enabled the transmission of signals across the damaged tissue, the Nature study reports.
If researchers can produce similar results in human studies, the findings could lead to a therapy to restore axon connections in people living with spinal cord injury.
“The idea was to deliver a sequence of three very different treatments and test whether the combination could stimulate disconnected axons to regrow across the scar in the injured spinal cord,” says lead author Michael Sofroniew (David Geffen School of Medicine, University of California Los Angeles, USA). “Previous studies had tested each of the three treatments separately, but never together. The combination proved to be the key.”
According to Sofroniew, many decades of research have shown that human nerve fibres need three things to grow: genetic programming to switch on axonal growth; a molecular pathway for the fibres to grow along; and a protein trail that entices the axons to grow in a particular direction. All three of these conditions are active when humans develop in the womb. After birth, these processes shut down, but the genes that control the growth programmes are dormant. Sofroniew’s goal was to re-start gene expression.
First, the researchers reactivated nerve cells in the spinal cords of mice by injecting a treatment packaged in a viral vector initially developed in the lab of Zhigang He (Harvard, Cambridge, USA).
Two weeks later, the UCLA team anesthetised the animals and disconnected the axons in their lower spinal cords. Only the rodents’ hind legs were affected and they could still move and feed.
Two days after injury, the team administered a second treatment into the lesion to create new pathways on which axons prefer to grow. Finally, the researchers released a third set of molecules called chemo-attractants. The axons target these chemo-attractants, and therefore the spinal cord tissue remaining on the other side of the scar from the injury.
When Sofroniew and his colleagues examined the tissue of mice who underwent the three-part treatment, they were jubilant. “Not only had axons grown robustly through the scar tissue,” Sofroniew recalls, “but many fibres had penetrated into the remaining spinal cord tissue on the other side of the lesion and made new connections with neurons there.”
Animals who did not undergo the combined treatment exhibited no axon regrowth across the injury lesion.
To test the reproducibility of their findings, the team repeated the experiment multiple times in mice at UCLA and in rats in the lab of Swiss neuroscientist Gregoire Courtine (Swiss Federal Institute of Technology Lausanne, Lausanne, Switzerland). The results proved equally robust.
Sofroniew and colleagues received another surprise when they tested whether newly regrown axons could conduct electrical activity in live animals. “When we stimulated the animal’s spinal cord with a low electrical current above the injury site, the regrown axons conducted 20% of normal electrical activity below the lesion,” comments Sofroniew. “In contrast, the untreated animals exhibited none.”
Despite the finding suggesting that the newly formed connections can conduct signals across the injury, the rodents’ ability to move did not improve. This was not unexpected, according to Sofroniew.
“We expect that these regrown axons will behave like axons newly grown during development—they do not immediately support coordinated functions,” explains Sofroniew. “Much like a new-born must learn to walk, axons that regrow after injury will require training and practice before they can recover function.”
The research team will next explore how to retrain newly wired circuits to restore movement.
This research was supported by the National Institute of Neurological Disorders and Stroke, the Dr. Miriam and Sheldon G. Adelson Medical Foundation, the International Foundation for Research in Paraplegia; ALARME Foundation, Association Song Taaba, Craig H. Neilsen Foundation, the European Research Council, Paralyzed Veterans Foundation of America, Swiss National Science Foundation, Microscopy Core Resource of UCLA Broad Stem Cell Research Center; Microscopy Core Resource of the Wyss Center for Bio and Neuroengineering; and Wings for Life.













